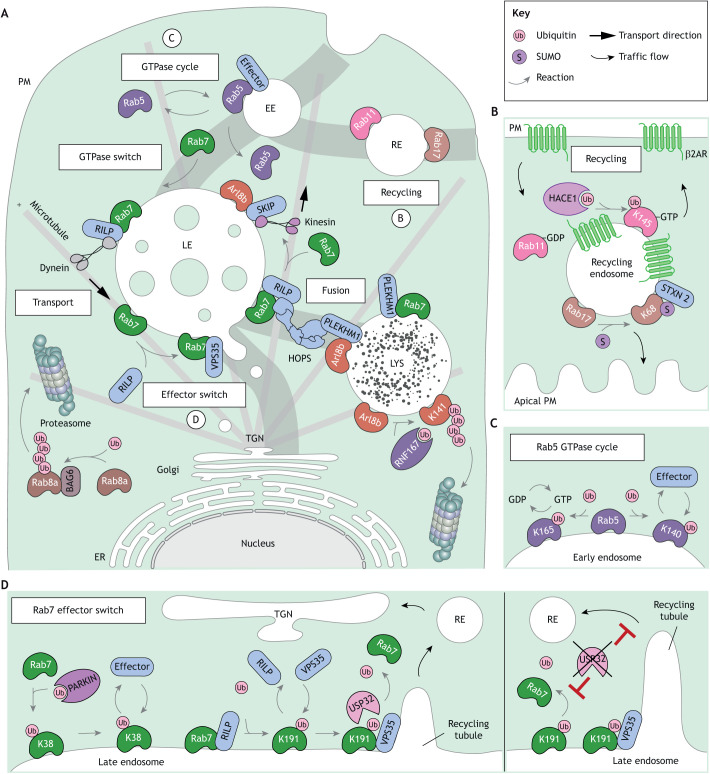
Fig. 3.
Ub and Ubls in endosomal membrane identity transitions. (A) Regulated switches between small GTPases determine endosome identity, transport and fusion through selective effector recruitment. During maturation, late endosome (LE)-associated Rab7 displaces early endosome (EE)-associated Rab5. Rab7 engages the effector RILP and the dynein motor to direct transport of late endosomes (LEs) to the perinuclear region. Here, HOPS-mediated tethering and fusion with lysosomes carrying Rab7 and/or Arl8b in complex with the effector PLEKHM1 takes place. On the other hand, displacement of Rab7 by Arl8b in complex with effector SKIP allows for kinesin motor-dependent transport to the cell periphery. Polyubiquitylation of Arl8b by E3 ligase RNF167 leads to its degradation by the proteasome. Other small GTPases including Rab8a are also turned over by the proteasome in a manner dependent on the cytosolic chaperone BAG6. Processes labeled B–D are shown in more detail in the other panels. (B) Ub and Ubl conjugation regulates Rab-mediated recycling. Endocytosed β2-adregergic receptor recruits the E3 ligase HACE1 to ubiquitylate and activate Rab11 for recycling to the plasma membrane (PM). Also, SUMOylation of Rab17 promotes SNARE syntaxin 2-mediated recycling to the apical surface. (C) Key consequences of GTPase ubiquitylation include altered GTP hydrolysis rate and effector interactions. This is illustrated by Rab5 whose monoubiquitylation on different lysine residues affects distinct aspects of its functional cycle. (D) Left, reversible ubiquitylation of Rab7 by the E3 ligase Parkin and DUB USP32 regulates effector preference. Monoubiquitylation of Rab7 disfavors interaction with RILP and promotes association with the retromer complex. Right, loss of USP32 inhibits recycling tubule resolution and blocks endosomes-to-TGN traffic. Acceptor lysine residues are indicated within some shapes.