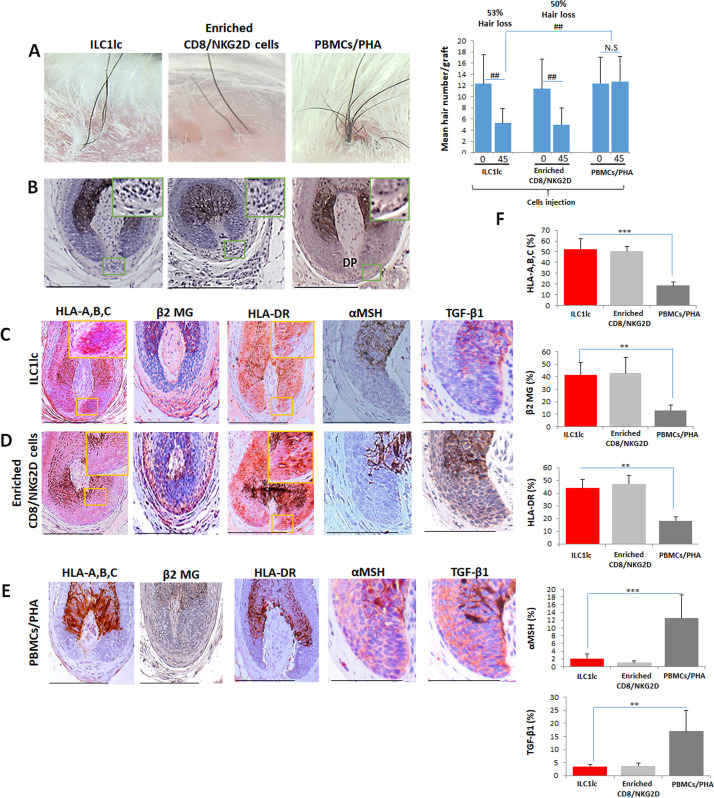
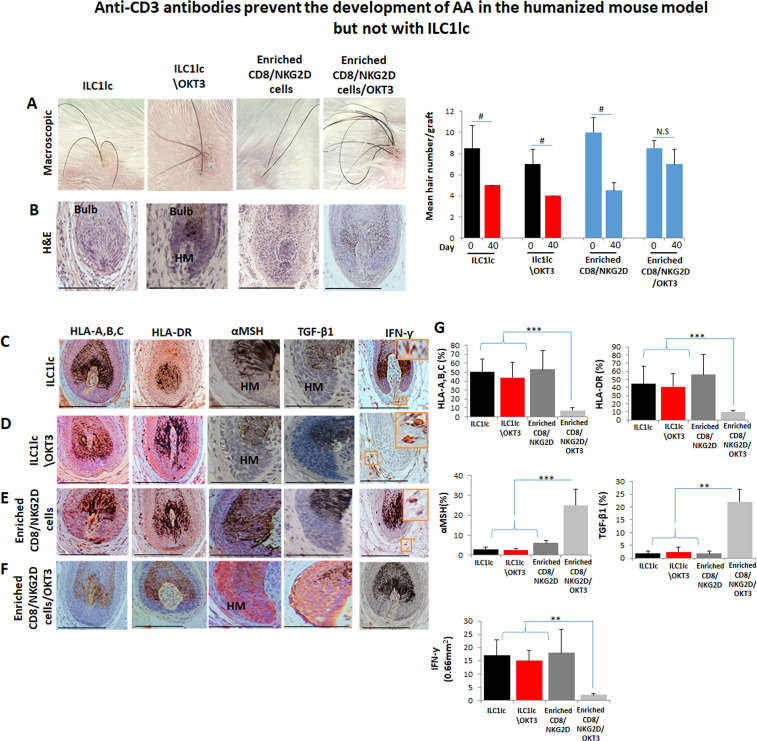
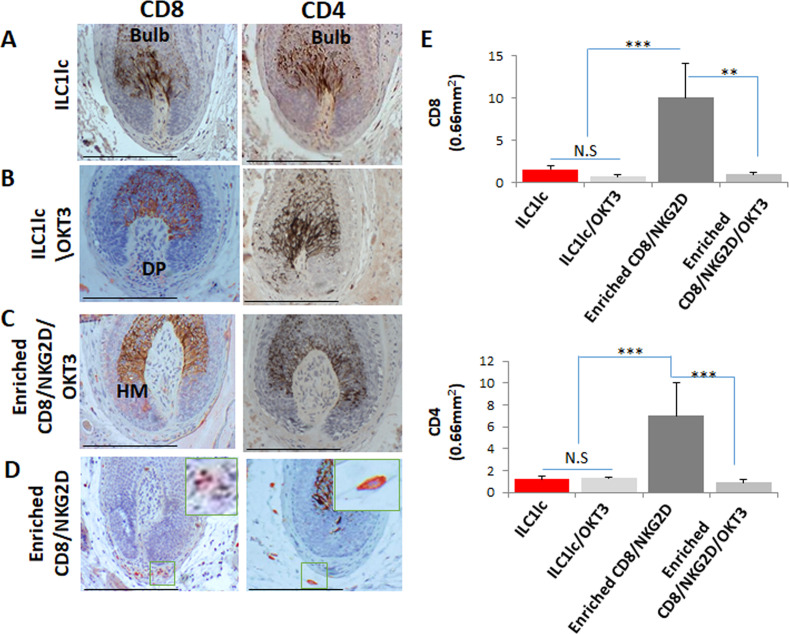
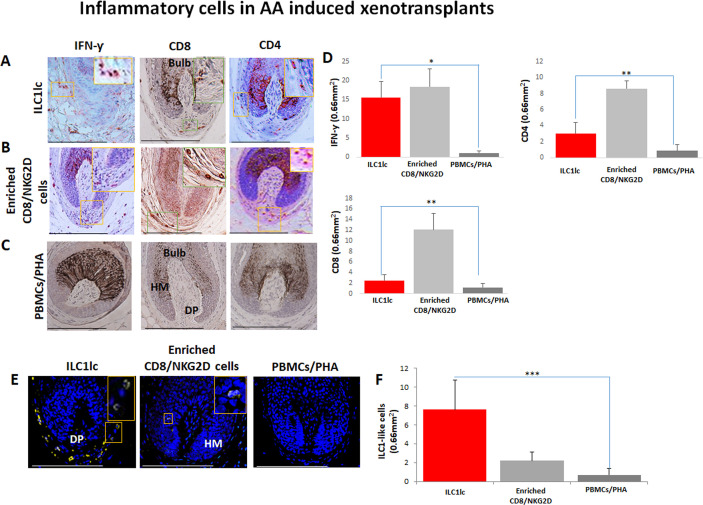
Figure 7. Development of alopecia areata (AA) in the humanized mouse model treated with ILC1lc.
(A) Significant hair loss is observed following the injection of ILC1lc and enriched CD8/NKG2D cells, while in the PBMCs/PHA treated group, hair number remains almost constant. N=7–9 xenotransplants/group from three independent donors, Following Shapiro-Wilk test, Mann-Whitney U test: #p<0.05, ##p<0.01. (B) HF dystrophy and perifollicular lymphocytic infiltrates around anagen hair follicles (HFs) (H&E staining) combined with strong expression of (C) HLA-A,B,C, β2 MG, HLA-DR, and downregulation of α-MSH and TGF-β1 in the ILC1lc and in (D) enriched CD8/NKG2D cells versus xenotransplants treated with (E) PBMCs/PHA (IHC staining) (F) quantitative data. N=5–9 xenotransplats/group from three independent donors. 4–5 defined reference areas were evaluated per section, and three sections per xenotransplants. Following Shapiro-Wilk test, Student’s t-test: *p<0.05, **p<0.01, ***p<0.001. Scale bar, 50 µm. DP - dermal papilla, HM - hair matrix.