Figure 2. Model for cross-priming CD8 T cells to immune evasive viruses through noncanonical cross-presentation.
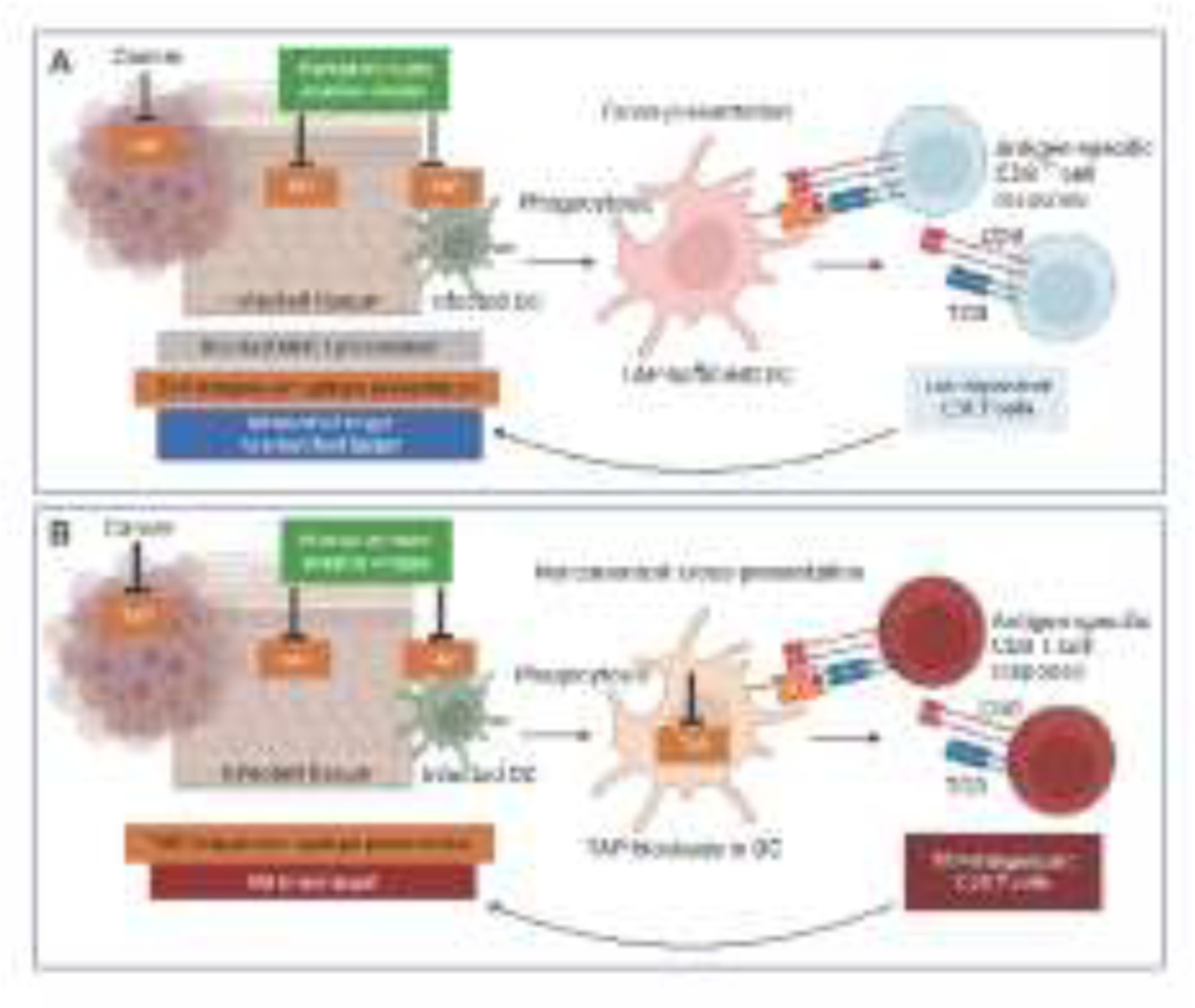
A. It is widely believed that priming a CD8 T cell response against immune evasive viruses such as Herpesviridae and Poxviridae that block TAP, can only be achieved by uninfected DCs which acquire viral peptides from internalized infected dying cells and present them to T cells. This solution accommodates the paradigm that CD8 T cell immunity is impaired without TAP. However, the presentation of TAP-dependent peptides is severely reduced on tissues infected with immune evasive viruses or in cancers that downmodulate TAP, and instead those tissues or cancers present peptides independently of TAP. Thus, the MHC-I peptidome on these tissues or cancers presents either a diminished or a mismatched target for TAP-dependent CD8 T cells primed by TAP-sufficient DCs leading to inefficient CD8 T cell targeting of infected or cancer cells. B. Noncanonical cross-presentation intrinsic to DCs that have compromised TAP function enables the cross-presentation of epitopes generated in the absence of TAP function to CD8 T cells. The resultant TAP-independent CD8 T cell response is best matched for targeting TAP-independent epitopes presented by tissues infected with immune evasive viruses or in cancers that downmodulate TAP. Mobilization of noncanonical cross-presentation can thus have critical impact on the design of CD8 T cell vaccines to immune evasive viruses and successful immunotherapy for immune evasive cancers.
