Figure 3. Subcellular localization and traffic of MHC-I molecules during noncanonical cross-presentation of microbial or self antigens.
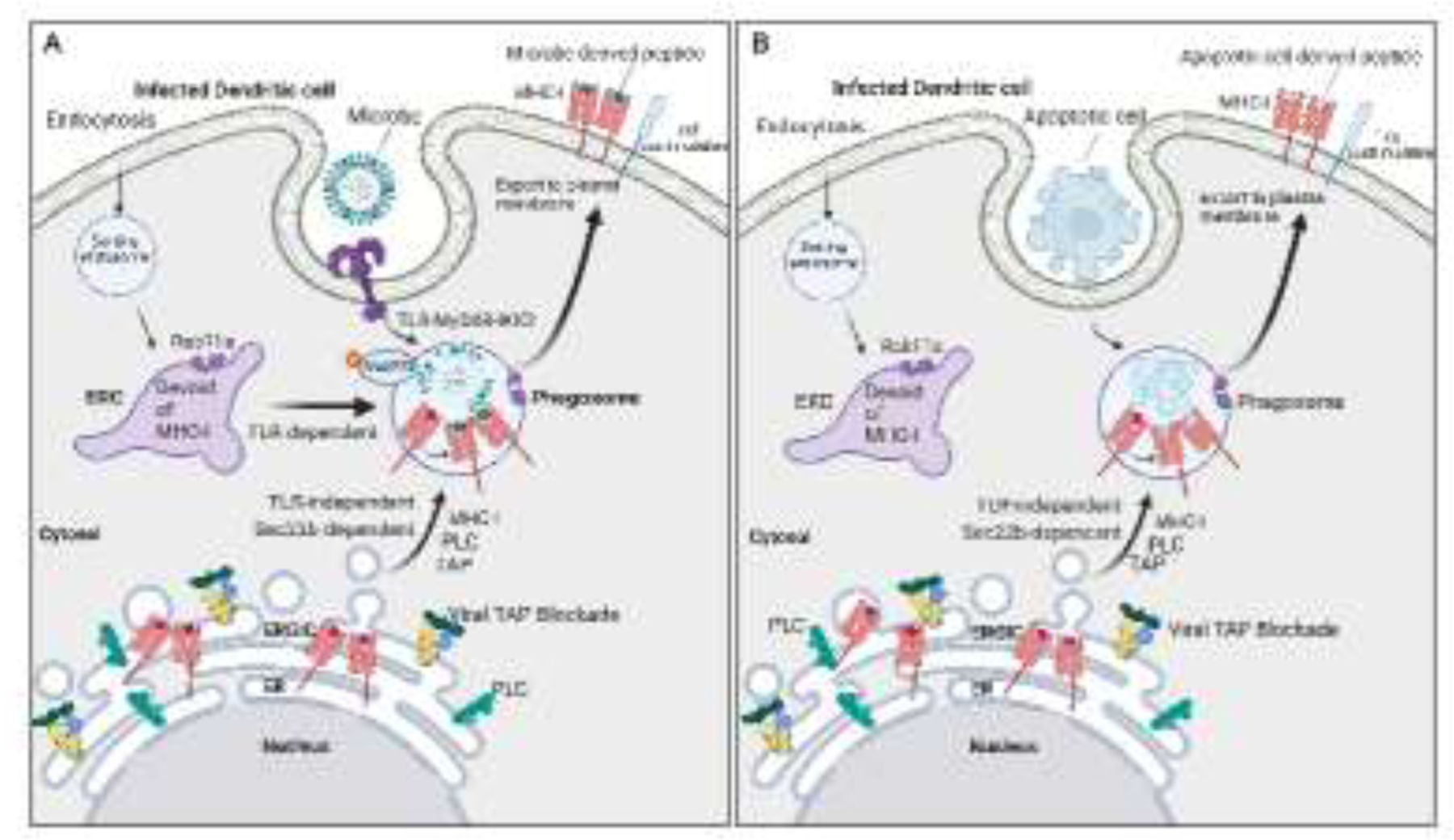
A. The ERC, which normally harbors large numbers of MHC-I molecules in DCs, becomes depleted of MHC-I molecules upon blockade of TAP. While TLRs continue to mobilize ERC-to-phagosome traffic, this traffic can no longer deliver MHC-I to phagosomes negatively impacting TLR-regulated cross-presentation. Under these conditions, DCs traffic MHC-I to phagosomes from the ERGIC instead of the ERC, in a manner dependent on the ER-SNARE protein Sec22b, to restore the MHC-I presentation of exogenously derived microbial peptides and rescue CD8 T cell priming. Because of the reliance on abnormal traffic of MHC-I, this pathway of antigen presentation is named as noncanonical cross-presentation. B. Noncanonical cross-presentation escapes regulation by TLR signals because it relies on trafficking MHC-I to phagosomes from the ERGIC, where they abnormally accumulate upon TAP blockade. Because TLRs do not control Sec22b-mediated traffic from the ERGIC to phagosomes, noncanonical cross-presentation carries the risk of cross-presenting peptides derived from phagocytosed apoptotic cells to self-reactive CD8 T cells in the context of infection-induced T cell costimulation.
