Figure 4. Metabolic Plasticity in Metastasis.
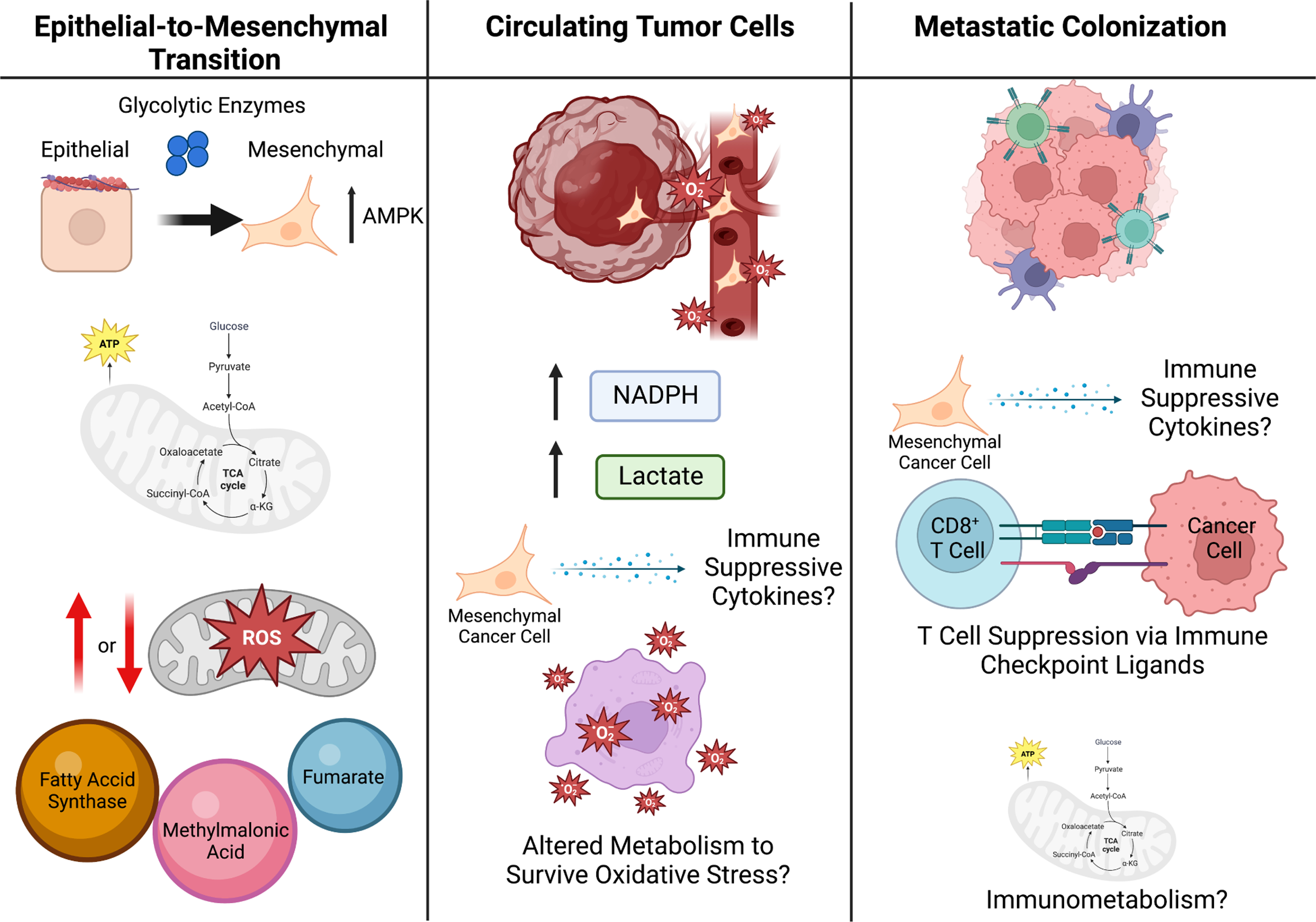
To escape the primary tumor and form metastases, cells undergo EMT (left panel). Glycolytic enzymes are attached to the cell cytoskeleton, as such it is possible that during cytoskeleton rearrangement in the EMT process, these glycolytic enzymes are released to promote glycolysis during EMT. The production of mitochondrial ROS within the cancer cells has been suggested to both promote and reduce EMT and metastatic potential of cancer cells, highlighting the likelihood that this process is highly dependent on the genetic context and local TME. Additionally, some metabolites act as pro-EMT signaling molecules, such as fumarate, methylmalonic acid, and fatty-acid synthase (FASN). Once in circulation (middle panel), cancer cells undergo oxidative stress and thus cell-death, therefore it is likely the cancer cells that are able to survive oxidative stress have a metabolic advantage resulting in their survival. Additionally, CTCs have been shown to upregulate NADPH production and lactate uptake, which diverts glucose carbon into the oxidate PPP and increases their antioxidant capacity. It is likely that cytokines and metabolites within the blood stream may influence both immune surveillance of CTCs as well as cancer cell immune evasion. Once cancer cells enter the metastatic site (right panel), they must act on the tumor microenvironment within the metastatic site to suppress immune surveillance, which can be done metabolically, such as by presenting immune checkpoint ligands. Additionally, immunometabolism likely plays a significant role in this process and may provide a therapeutic vulnerability for the treatment of metastatic cancer.
