Abstract
Context:
Early, concurrent palliative care interventions in chronic obstructive pulmonary disease (COPD) are limited. Project EPIC (Early Palliative Care In COPD) is a multiphase mixed methods study working to fill this gap.
Objective:
To conduct a formative and summative evaluation of EPIC, a telephonic nurse coach-led early palliative care intervention for COPD adapted from the ENABLE intervention in cancer.
Methods:
Phase I Formative Evaluation: Patients with moderate-to-very-severe COPD, family caregivers, and pulmonary and palliative care clinicians rated the acceptability and feasibility of EPIC (≥4 out of 5 on a Likert-scale survey). Phase II Summative Evaluation: Patients and family caregivers in Phase I participated in a pilot of the 3-month EPIC prototype to evaluate intervention and data collection feasibility (≥70% completion) and to seek qualitative feedback.
Results:
Phase I Formative Evaluation: Patients (n=10), family caregivers (n=10), pulmonary clinicians (n=6), and palliative care clinicians (n=6) found EPIC acceptable and feasible to support adaptation, while priority early palliative care needs in COPD from our prior research mapped well to the EPIC prototype. Phase II Summative Evaluation: Patients (n=5; ages 49-72, 40% moderate COPD, 40% Black) and their family caregivers (n=5; ages 51-73, 40% Black) completed 100% of EPIC prototype components, including weekly telephone sessions, a 1-month follow-up call, Advance Directive, palliative care clinic attendance, and 95% of monthly phone data collection sessions. Feedback from participants about EPIC was all positive.
Conclusions:
EPIC was acceptable and feasible in patients with COPD and their family caregivers. Larger feasibility and effectiveness trials are warranted.
Keywords: palliative care, COPD, chronic obstructive pulmonary disease, family caregiver, intervention development, implementation science, mixed methods, qualitative, pilot study
INTRODUCTION
The United States (U.S.) has seen an increase in specialist palliative care access over the past two decades, though multiple barriers still exist to ambulatory palliative care, especially in rural areas.1 Data have demonstrated palliative care’s positive impact on people living with chronic obstructive pulmonary disease (COPD), through more home deaths, more goal-concordant end-of-life care, and reduction in healthcare utilization near the end of life.2 However, its integration in COPD is rare, and most referrals to specialist palliative care for people living with COPD in the U.S. occur late and near the end-of-life.3-5 This leaves a missed opportunity to positively impact COPD outcomes and address upstream patient and family caregiver needs through early, concurrent palliative care.3,6
A successful model of early, concurrent palliative care that demonstrated effectiveness in patients with advanced cancer is ENABLE© (Educate, Nurture, Advise, Before Life Ends). First developed in 1999, ENABLE is a telephonic early concurrent palliative care intervention for patients with advanced cancer and their family caregivers.7,8 ENABLE is based on Wagner’s Chronic Care Model and focuses on activating patients and their family caregivers to engage with clinicians and health systems to improve their quality of life and coping skills.9 ENABLE improved quality of life and mood in patients with advanced cancer and their family caregivers, improved patient survival, and has been adapted for heart failure.7,10,11 ENABLE uses a telephonic nurse coaching delivery model and a manualized curriculum [Charting Your Course (CYC)] to activate patients and their family caregivers in developing skills to support problem solving, symptom recognition and management, communication, and decision-making.
Our qualitative data revealed that people living with COPD have significant early palliative care needs, priority among them being coping with COPD, emotional symptoms, respiratory symptoms, illness understanding, and prognostic awareness.12 Further, these needs exist in patients with less severe COPD, e.g. Global Initiative for Chronic Obstructive Lung Disease (GOLD) Stage II, a group not typically included in palliative care clinical trials. We also found that family caregivers of patients with COPD shared many early palliative care needs.12 Because of these needs and the demonstrated successes of ENABLE in advanced cancer,12-15 we conceptualized Project EPIC: (Early Palliative Care In COPD) based on the core content, format, and delivery model of ENABLE.
Our present efforts are focused on developing the EPIC intervention, adapted from the evidence-based ENABLE intervention, and exploring its feasibility in patients with COPD and their family caregivers. This paper describes two phases of Project EPIC: 1) Phase I formative evaluation to support acceptability and intervention adaptation; and, 2) Phase II summative evaluation to pilot test the EPIC prototype. We had three primary research questions: 1) Do patients with COPD, their family caregivers, and clinicians find the EPIC intervention acceptable and potentially feasible for implementation in COPD?; 2) Does EPIC address early palliative care needs in COPD?; and, 3) Can the EPIC intervention prototype and data collection protocol be feasibly completed in patients and their family caregivers?
METHODS
Study Design
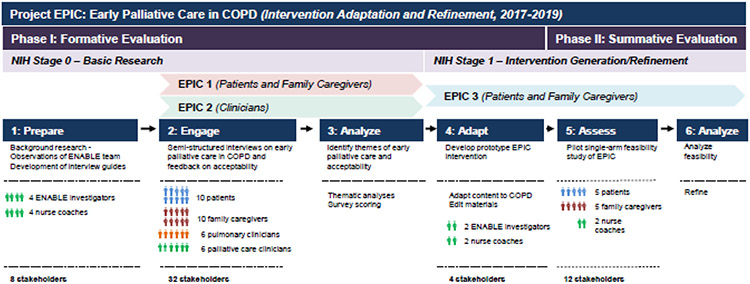
Project EPIC is a multiphase formative (Phase 1) and summative (Phase 2) evaluation study guided by the NIH Stage Model for the development of complex behavioral interventions (Figure 1). Project EPIC is part of an ongoing evaluation of ENABLE across serious illnesses and among patient and family caregiver populations.7,11 Between 2017-2019, Project EPIC advanced from NIH Stage 0 (Basic Research) to Stage 1 (Intervention Generation/Refinement). As illustrated in Figure 1, we followed a human-centered design approach (Prepare → Engage → Analyze → Adapt → Assess → Analyze) to engage a diverse group of participants across many phases of intervention development.16,17 We adhered to COREQ reporting guidelines (Supplemental Table 1). Our study was approved by the Institutional Review Board at the University of Alabama at Birmingham (IRB-170209003).
Figure 1: Project EPIC Multiphase Formative and Summative Evaluation.
Project EPIC is a multiphase study that follows the NIH Stage Model for the development of complex behavioral interventions and is part of an ongoing evaluation of ENABLE across serious illnesses. This figure illustrates the Phase I formative evaluation and Phase II summative evaluation of Project EPIC. The figure shows the specific NIH Stages and the different EPIC studies that were conducted: EPIC 1 included patients and family caregivers; EPIC 2 involved clinicians; and, EPIC 3 was a summative evaluation/pilot study with patients and family caregivers. We followed a human-centered design approach to engage a diverse group of stakeholders from “Prepare” to “Assess” phases. Abbreviations: EPIC = Early Palliative Care in COPD
Phase I Eligibility and Setting
Phase I began with the PI attending weekly ENABLE study team meetings as an immersion into team processes and study protocol. This was followed by EPIC 1 and EPIC 2 (Figure 1). EPIC 1 engaged patients and their family caregivers, and EPIC 2 involved pulmonary and palliative care clinicians. Details of EPIC 1 and EPIC 2 eligibility criteria, methods, and results have been published previously.12,18 Briefly, Phase I patient participants were adults >40 years with moderate to very severe COPD [Forced Expiratory Volume in 1 second (FEV1) /forced vital capacity (FVC) <0.70 and FEV1 <80%] who either had severe dyspnea [modified Medical Research Council Dyspnea Scale (mMRC) >2], or were on supplemental oxygen, or had a severe exacerbation of COPD that required hospitalization in the previous year. Patient participants were required to have a family caregiver described as an “unpaid family member or close relative >19 years who was integral to providing care for them on a daily basis”. Patient participants were excluded if they had another primary pulmonary condition, lung cancer, serious mental illness, or cognitive impairment by a 6-item screener. Participants were recruited from pulmonary clinics at the University of Alabama at Birmingham (UAB) and Cooper Green Mercy Health Services in Birmingham, AL. Clinicians were UAB pulmonary and palliative care clinicians (physicians and nurse practitioners).12,18 Phase I patient participants completed written informed consent, while their family caregivers and clinicians were given an information sheet and provided verbal consent.
Phase I Procedures
Phase I patient, family caregiver, and clinician participants completed semi-structured indepth interviews to explore priority early palliative care needs in COPD; the results of the thematic analysis from these interviews have been published elsewhere.12 To support adaptation of ENABLE to EPIC, we sought to understand how participants supported ENABLE and the EPIC intervention. After completing in-person semi-structured interviews on needs and challenges, we briefly described the structure of the ENABLE program and reviewed printed copies of guidebooks. We then asked participants to complete an acceptability and feasibility survey (Supplemental Table 2) to seek their feedback on the ENABLE program and its adaptation to COPD as EPIC. We developed a 9-question Likert-scale survey, with each question graded on a 5-point scale (5 being the best) to determine their initial reaction to a description of the ENABLE program, to seek feedback on acceptability and feasibility of its adaptation to COPD as EPIC, to rate ENABLE printed materials, to assess their potential for participation in EPIC, and to seek feedback on proposed data collection instruments. We recorded data in REDCap and reported participants’ demographic and clinical characteristics as means (±standard deviation) for continuous variables and frequency (%) for categorical variables. We a priori defined scores of ≥4 out of 5 on each question as evidence of acceptability and feasibility to support adaptation. Phase I participants received $25 after completing the interview and survey. We used SPSS Version 23 for all statistical analyses.
Phase II Prototype Adaptation and Development
Phase II began with EPIC 3, which consisted of EPIC prototype development and pilot testing (Figure 1). We used data from the Phase I survey on acceptability and feasibility to support commencement of intervention adaptation from ENABLE to EPIC and then mapped our previously reported data on priority early palliative care needs in COPD 12 to the EPIC prototype. Intervention adaptation involved translating ENABLE activities and intervention components from cancer to COPD. Examples included refining an activity on problem solving from fatigue to breathlessness and adapting topics to focus on COPD-specific symptoms. We identified and refined resources specific for COPD throughout the intervention. Finally, we revised all nurse coach transcripts and tailored aspects of advance care planning sections to COPD, e.g. invasive mechanical and non-invasive ventilation.
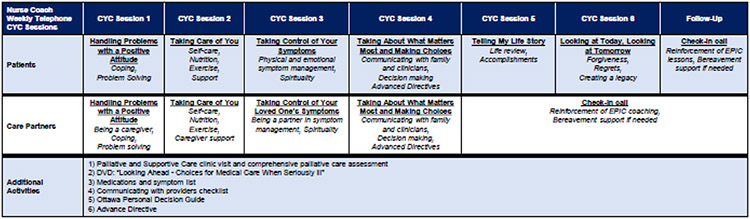
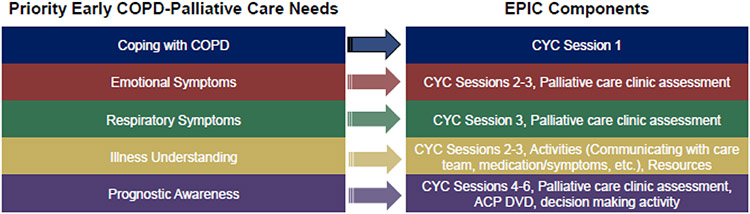
The end product was an EPIC prototype that harnessed ENABLE’s telephonic nurse-coaching guided by the CYC curriculum, with six telephone sessions for patients and four telephone sessions for family caregivers (Figure 2). Sessions 1-3 of the CYC curriculum focused on McMillan’s COPE (Creativity, Optimism, Problem Solving, Expert information) Model and guided patients through problem solving, self-care, symptom management, and decision-making.19 Sessions 4-6 implemented Steinhauser’s OUTLOOK model for life review and creating a legacy.20 At the beginning of each patient CYC session, nurse coaches screened for distress using the National Comprehensive Cancer Network Distress Thermometer, which ranges from 0 to 10 and has been validated in COPD.21 For patient participants with scores ≥3, nurse coaches discussed sources of distress and helped identify ways to resolve problems.22 Additional EPIC components included a DVD on advance care planning (HealthAnalog), activities within each CYC session, and attendance at a specialist palliative and supportive care clinic to complete a comprehensive assessment informed by National Consensus Project guidelines (Figure 2).23 Our nurse coaches communicated with clinic administrators using the electronic medical record to schedule this appointment. A telephone call one month after completing the CYC sessions focused on maintenance and reinforcement of content and lessons. The entire EPIC prototype, including the 1-month follow-up call, was designed to be completed in three months. As shown in Figure 3, priority early palliative care needs in COPD from our previous research in EPIC 112 mapped well to the EPIC prototype intervention.
Figure 2. Prototype EPIC Intervention Components.
This figure illustrates the components of the prototype EPIC intervention. EPIC is a telephonic, nurse-coach led early palliative care for patients with COPD and their family caregivers and adapted from the ENABLE model for early palliative care in advanced cancer. EPIC uses the Charting Your Course curriculum and printed guidebooks. Patients complete six telephone sessions, and family caregivers complete four sessions with separate nurse coaches. Additional activities include attending an in-person palliative and supportive care clinic visit and completing an Advance Directive. Abbreviations: CYC = Charting Your Course
Figure 3. Mapping Priority Early Palliative Care Needs in COPD to EPIC.
This figure illustrates how priority early palliative care needs from our previous qualitative research on the left mapped to components of the EPIC prototype on the right. Abbreviations: CYC = Charting Your Course; ACP = Advance care planning
For this pilot study, we recruited two nurse coaches from the experienced pool of ENABLE nurse coaches who were registered nurses with backgrounds in palliative care. One was certified in hospice and palliative nursing, and the other was completing a Master of Science in Nursing with a subspecialty in advanced palliative care. ENABLE nurse coaches received palliative care training with palliative care clinicians and experts (MB, JND-O, RT, and RDW), and the robust palliative care training for ENABLE coaches has been detailed previously.24 Additionally, the PI (ASI) provided nurse coaches with literature on early palliative care needs in COPD and discussed these topics with them.
Phase II Prototype Pilot Testing (Eligibility, Setting, and Procedures)
The next stage of EPIC 3 was a single-arm pilot study of the EPIC intervention prototype (Figure 1). For this pilot, we purposively recruited a sample of five patient and their family caregiver participants from Phase I. Nurse coaches recorded completion of all intervention components. Consistent with our prior research,7,13,14 we a priori defined EPIC prototype feasibility as completion of ≥70% of intervention components. Nurse coaches took detailed field notes of each telephone session for review with the PI at weekly team meetings. Fidelity focused on these topics: progress, potential challenges, concerns, burdensome symptoms, and care needs. Upon completion of the final intervention telephone session (CYC session six for patients and session four for family caregivers), nurse coaches recorded feedback from participants on their perceived benefit of the program and potential areas of improvement. We reviewed these field notes and collected exemplary quotes.
Our data collection instruments were collected by phone at baseline and monthly for up to four months and captured a broad array of symptoms, quality of life, Life-Space mobility, and family caregiver burden data, as detailed in Supplemental Table 3. The primary goal at this stage was to record data collection feasibility, defined a priori as completion of ≥70% of instruments, in preparation for the next stage. Phase II patient and family caregiver participants each provided written informed consent to participate and received $10 for each intervention session completed as well as $10 for each data collection session. Data collection and analyses were conducted in a similar fashion as in Phase I.
RESULTS
Phase I Formative Evaluation (EPIC 1 and EPIC 2; NIH Stage 0 - Basic Research)
Phase I Cohort Characteristics
Phase I participants (N=32) included patients, family caregivers, pulmonary clinicians, and palliative care clinicians (Table 1). Patients (n=10) were a mean age (±SD) of 60.4±7.5 years, and 50% were Black. Participants represented a variety of COPD severity stages. Family caregivers (n=10) were a mean age of 58.3±8.7 years, and 40% were Black. Clinicians (n=12) were a mean age of 45.9±15.4 years, with 90% White and equal distribution between palliative care and pulmonary specialties.
Table 1.
Phase 1 Formative Evaluation (EPIC 1 and EPIC 2 Participant Characteristics) (n=32)
| Characteristics | Patients (n=10) |
Family Caregivers*
(n=10) |
Clinicians (n=12) |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (Years, mean±SD) | 60.4±7.5 | 58.3±8.7 | 45.9±15.4 |
| Race (African American) (%) | 5 (50) | 4 (40) | 0 (0) |
| Gender (Male) (%) | 7 (70) | 1 (10) | 9 (75.0) |
| Married (%) | 3 (30) | 5 (50) | n/a |
| Education (High School Graduate) (%) | 5 (50) | 5 (50) | n/a |
| Health Insurance (%) | |||
| Medicare | 5 (50) | 5 (50) | n/a |
| Uninsured | 3 (30) | 1 (10) | n/a |
| Current Smoking (%) | 5 (50) | 4 (40) | n/a |
| Religious Preference | |||
| Protestant (%) | 1 (10) | 3 (30) | n/a |
| None (%) | 3 (30) | 1 (10) | n/a |
| Regularly Attends Religious Services | 1 (10) | 7 (70) | n/a |
| Ever Prayed for Own Health | 7 (70) | 9 (90) | n/a |
| Prior Palliative Care | 1 (10) | n/a | n/a |
| Has Advance Directive | 1 (10) | n/a | n/a |
| Identified Surrogate Decision Maker | 3 (30) | n/a | n/a |
| Clinical Characteristics | |||
| Charlson Comorbidity Index† (mean±SD) | 1.5±1.0 | n/a | n/a |
| GOLD Stage‡ | |||
| GOLD II (Moderate) (%) | 3 (30) | n/a | n/a |
| GOLD III (Severe) (%) | 3 (30) | n/a | n/a |
| GOLD IV (Very Severe) (%) | 4 (40) | n/a | n/a |
| FEV1 (%-Predicted, mean±SD | 37.4±20.4 | n/a | n/a |
| Supplemental Oxygen (%) | 8 (80) | n/a | n/a |
| ≥1 Severe Exacerbations in the Year Prior (Hospital or Emergency Room) (%) | 3 (30) | n/a | n/a |
| Prior Cardiopulmonary Rehabilitation (%) | 4 (40) | n/a | n/a |
| Severe Breathlessness§ | 8 (80) | n/a | n/a |
| Clinician Characteristics | |||
| Specialty | |||
| Pulmonary-Critical Care | n/a | n/a | 6 (50.0) |
| Palliative Care | n/a | n/a | 6 (50.0) |
| Discipline | |||
| Physician | n/a | n/a | 10 (83.3) |
| Nurse Practitioner | n/a | n/a | 2 (16.7) |
| Academic Rank | |||
| Fellow | n/a | n/a | 1 (8.3) |
| Instructor | n/a | n/a | 2 (16.7) |
| Assistant Professor | n/a | n/a | 5 (41.7) |
| Associate Professor | n/a | n/a | 3 (11.5) |
| Professor | n/a | n/a | 1 (3.8) |
Care partners represented the following relationships with patients: spouse (n=4), ex-spouse (n=2), significant other (n=2), child (n=1), parent (n=1)
Derived from 19 comorbidities; higher points associated with higher mortality.
Moderate = 50% ≤ FEV1 <80%; Severe: 30% ≤ FEV1 <50%; Very Severe: FEV1 <30%
≥2 on the Modified Medical Research Council scale for dyspnea
Abbreviations: GOLD = Global Initiative for Chronic Obstructive Lung Disease; FEV1 = Forced expiratory volume in 1 second
Phase I Acceptability/Feasibility
As shown in Table 2, participants (N=32) across stakeholder groups found the ENABLE program and our proposed adaptation to EPIC as acceptable and feasible (average scores across each group ≥4 out of 5, Table 2). Clinician participants rated the likelihood of attending an in-person palliative care visit lower than patient and family caregiver participants (mean scores: 4.4±0.5 for patients and 4.6±0.7 for family caregivers versus 3.5±0.8 for pulmonary clinicians and 3.2±0 for palliative care clinicians). Patient and family caregiver participants rated the proposed data collection instruments as feasible (mean score 4.6±0.5 for patients and 4.8±0.6 for family caregivers).
Table 2.
Phase I Formative Evaluation (EPIC 1/EPIC 2 – Participant Acceptability/Feasibility) (n=32)
| Survey Content | Patients (n=10) |
Family Caregivers (n=10) |
Pulmonary Clinicians (n=6) |
Palliative Care Clinicians (n=6) |
|---|---|---|---|---|
| Initial reaction to the ENABLE program as described | 4.5±0.7 | 4.3±0.8 | 4.5±0.8 | 4.5±0.5 |
| Acceptability of EPIC for patients and their family caregivers | 4.8±0.4 | 4.7±0.5 | 4.5±0.8 | 4.5±0.8 |
| Feasibility of the EPIC program in patients with COPD and their family caregivers | 5.0±0.0 | 4.9±0.4 | 4.2±0.4 | 3.8±1.2 |
| Design of ENABLE guidebooks, i.e. pictures, printed text, readability | 4.5±0.5 | 4.2±0.8 | 4.2±1.0 | 4.3±0.8 |
| Likelihood of attending an in-person palliative care clinic visit | 4.4±0.5 | 4.6±0.7 | 3.5±0.8 | 3.2±0 |
| Likelihood of attending a telehealth video palliative care clinic visit | 4.0±1.3 | 4.0±1.5 | 3.3±1.3 | 3.5±0.5 |
| Likelihood of participating in EPIC (patients & family caregivers) | 4.7±0.5 | 4.6±0.7 | n/a | n/a |
| Likelihood of recommending EPIC to their patients and other clinicians (clinicians) | 4.8±0.4 | 4.9±0.3 | 4.7±0.8 | 5.0±0.0 |
| Feasibility of proposed data collection instruments | 4.6±0.5 | 4.8±0.6 | n/a | n/a |
Note: Questions scored on a Likert scale from 1 to 5, with 5 being the highest/best score.
Phase II Summative Evaluation (EPIC 3 Pilot Study; NIH Stage I - Intervention Generation/Refinement)
Phase II Cohort Characteristics
Phase II participants and included five dyads of patients and their family caregivers from Phase I. Patients (n=5) had a mean age of 60.2±10.6 years (range 49-72 years), with 60% White, 40% male, and 40% GOLD II-III COPD stages. Family caregivers (n=5) had a mean age 59.0±8.9 years (range 51-73 years), with 40% White and 20% male (Table 3). Most patient participants in Phase II were on supplemental oxygen (n=4), and none had prior experience with palliative care. Only one dyad had previously completed an Advance Directive. The majority of family caregivers (n=3) were patient participants’ spouses. Baseline data reveal that both patient and family caregiver participants had poor quality of life across multiple domains (Table 3).
Table 3.
Phase II Summative Evaluation (EPIC 3 Pilot Study Participant Characteristics at Baseline) (N=10)
| Characteristics | Patients (n=5) |
Family Caregivers * (n=5) |
|---|---|---|
| Demographic Characteristics | ||
| Age (Years) | 60.2±10.6 Range: 49-72 | 59.0±8.9 Range: 51-73 |
| Race (African American) (%) | 2 (40) | 2 (40) |
| Gender (Male) (%) | 2 (40) | 1 (20) |
| Married (%) | 1 (20) | 3 (60) |
| Relationship to Patient | ||
| Spouse | n/a | 3 (60) |
| Significant Other | n/a | 1 (20) |
| Child | n/a | 1 (20) |
| Education (>High School Graduate) (%) | 4 (80) | 5 (100) |
| Health Insurance (%) | ||
| Medicare | 3 (60) | 2 (40) |
| Medicaid | 1 (20) | 1 (20) |
| Uninsured | 1 (20) | 0 |
| Current Smoking (%) | 4 (80) | 3 (60) |
| Regularly Attends Religious Services | 0 | 3 (60) |
| Ever Prayed for Own Health | 3 (60) | 4 (80) |
| Prior Palliative Care | 0 | 0 |
| Identified Surrogate Decision Maker | 0 | 1 (20) |
| Advanced Directive | 1 (20) | 1 (20) |
| Clinical Characteristics | ||
| Charlson Comorbidity Index* | 3.6±1.3 Range: 2-5 | n/a |
| FEV1 (%-Predicted) | 39.2±29.0 Range: 13-71 | n/a |
| GOLD Stage† | ||
| GOLD II-III (Moderate-Severe) (%) | 2 (40) | n/a |
| GOLD IV (Very Severe) (%) | 3 (60) | n/a |
| Supplemental Oxygen (%) | 4 (80) | n/a |
| Prior Cardiopulmonary Rehabilitation (%) | 3 (60) | n/a |
| Baseline Participant Reported Outcomes | ||
| COPD Symptom Burden (Chronic Respiratory Questionnaire) | ||
| Breathlessness | 3.4±1.7 Range: 1.8-6.0 | n/a |
| Fatigue | 3.2±1.7 Range: 1.8-5.8 | n/a |
| Emotional Function | 4.5±1.3 Range: 3.4-6.4 | n/a |
| Mastery | 4.8±1.9 Range: 2.3-7.0 | n/a |
| Patient Activation (Patient Assessment of Chronic Illness Care) | 57.4±26.0 | n/a |
| Quality of Life/Emotional Symptoms (PROMIS Measures) | ||
| PROMIS Global Health Physical Function | 35.3±3.4 Range: 29.6-37.4 | 43.1±6.3 Range:34.9-50.8 |
| Poor Quality of Life (%) | 5 (100) | 3 (60) |
| PROMIS Emotional Distress - Anxiety | 55.4±10.5 Range: 37.1-63.5 | 55.2±8.6 Range: 47.8-68.7 |
| Moderate-Severe Anxiety Symptoms (%) | 2 (40) | 1 (20) |
| PROMIS Emotional Distress - Depression | 55.2±6.6 Range: 47.5-59.4 | 58.0±6.1 Range: 49.8-64.4 |
| Moderate-Severe Depression Symptoms | 1 (20) | 3 (60) |
| Mobility/Social Isolation (Life-Space Mobility) | ||
| UAB Life Space Assessment | 63.8±29.9 Range: 27.0-92.0 | 78.8±31.1 Range: 38.0-110.0 |
| Restricted Life-Space Mobility (%) | 2 (40) | 2 (40) |
| Dyadic Typology | ||
| Individual Management | 3 (60) | 1 (20) |
| Patient Oriented | 3 (60) | 1 (20) |
| Caregiver Oriented | 0 | 0 |
| Joint Management | 2 (40) | 4 (80) |
| Collaboratively Oriented | 0 | 1 (20) |
| Complementarily Oriented | 2 (40) | 3 (60) |
| Dyadic Arrangement Satisfaction | ||
| Extremely Unsatisfied | 1 (20) | 0 |
| Somewhat Unsatisfied | 0 | 1 (20) |
| Undecided | 1 (20) | 1 (20) |
| Somewhat Satisfied | 0 | 1 (20) |
| Extremely Satisfied | 3 (60) | 2 (40) |
| Dyadic Adjustment Scale | 27±3.4 Range: 24-31 | 17.6±7.4 Range: 6-25 |
| Baseline Care Partner Burden | ||
| Family Caregiver Impact | n/a | 49.2±7.0 |
| Family Caregiver Objective Burden | n/a | 21.4 (3.8) |
| High Family Caregiver Objective Burden (%) | n/a | 2 (40) |
| Family Caregiver Subjective Demand | n/a | 15.5±2.6 |
| High Family Caregiver Subjective Demand (%) | n/a | 2 (40) |
| Family Caregiver Subjective Stress | n/a | 15.6±3.0 |
| High Family Caregiver Subjective Stress (%) | n/a | 3 (60) |
| Positive Aspects of Caregiving | ||
| Total | n/a | 31.6±7.5 |
| Self-Affirmation | n/a | 18.2±4.3 |
| Outlook | n/a | 11.2±1.9 |
Derived from 19 comorbidities; higher points associated with higher mortality.
Moderate = 50% ≤ FEV1 < 80%; Severe: 30% ≤ FEV1 < 50%; Very Severe: FEV1 < 30%
Abbreviations: GOLD = Global Initiative for Chronic Obstructive Lung Disease; FEV1 = Forced expiratory volume in 1 second
Phase II Prototype & Protocol Feasibility
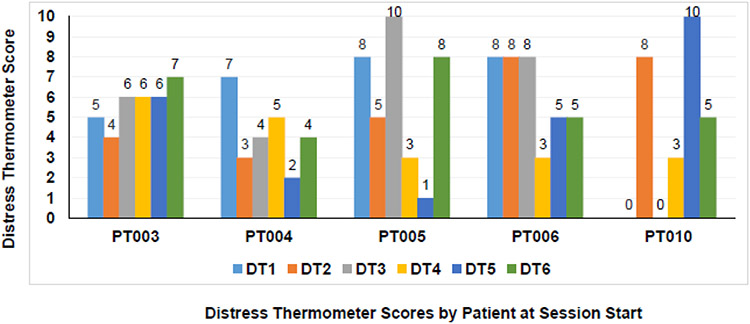
Table 4 shows the completion rates of the prototype EPIC components. Overall, patient and family caregiver participants completed 100% of the nurse coach CYC sessions (six for patients and four for family caregivers) and also completed 100% of the follow-up calls at one-month. All participants completed Advance Directives, viewed the DVD on advance care planning, and attended an in-person palliative and supportive care clinic appointment (Table 4). Patient participants completed their six CYC telephone sessions in 50±7 days on average, and family caregivers completed their four CYC sessions in 46±13 days. The average time for patient-family caregiver dyads to attend the in-person palliative and supportive care clinic appointment was 66±44 days from beginning the intervention. Patient participants completed 100% of the monthly data collection sessions (n=20 of 20 total sessions for all patient participants), while family caregivers completed 90% of these sessions (n=18 of 20 total sessions for all family caregiver participants). Figure 4 shows the pre-session distress screening scores over the six CYC sessions for patient participants. Four patient participants had high levels of distress (≥3) at the beginning of each CYC session, and distress was highly variable over time. Exemplary quotes from the field notes collected by nurse coaches at their final CYC session with participants are presented in Table 5. All patient and family caregiver participants supported the intervention, valued their time with nurse coaches, and described ways that they gained better illness understanding and prognostic awareness.
Table 4.
Phase II Summative Evaluation (EPIC Prototype Feasibility) (n=10)
| Dyad | Patient CYC Sessions* Completed |
Patient Follow-Up Call - Completed |
Family Caregiver CYC Sessions** Completed |
Family Caregiver Follow-Up Call- Completed |
In-Person Palliative Care Assessment Completed |
Viewed ACP DVD |
Completed Advance Directive |
Number of Fully Completed Patient Data Collection Sessions |
Number of Fully Completed Family Caregiver Data Collection Sessions |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 4/4 | 4/4 |
| 2 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 4/4 | 4/4 |
| 3 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 4/4 | 4/4 |
| 4 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 4/4 | 3/4*** |
| 5 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 4/4 | 3/4*** |
Mean (±SD) days to complete CYC sessions: Patients: 50 (7); Family caregiver: 46 (13)
Mean (±SD) days to complete CYC session plus 1 monthly follow up call: Patients: 80 (7); Family caregivers: 76 (13) Average time to clinic appointment: 66±44 days
Out of 6 sessions
Out of 4 sessions
The family caregivers in these dyads were unable to complete their fourth session (of four) due to challenges coordinating data collection around their busy job schedules.
Figure 4. Patient Participant Distress Thermometer Scores by Session.
This figure displays patient participant NCCN Distress Thermometer scores that occurred at the beginning of each of their CYC sessions. Each patient had a total of six distress screenings. Distress scores range from 0 to 10, with higher scores indicating more severe distress, and scores ≥3 indicating high distress. Abbreviations: DT = Distress Thermometer. PT = Patient
Table 5.
Patient and Family Caregiver Feedback on CYC Sessions from Nurse Coach Field Notes
|
“He feels like these sessions were like therapy since he is home bound. He likes the human contact. He states it is nice to talk about these things. He can' offer anything to help me do a better job. He has learned new things about himself in that he is stubborn.” (PT003 – 72yo White Male, FEV1 26% - very severe COPD) “She liked the intervention and thought it was helpful. She liked being able to take time out to look at stuff. She thought we could include more COPD caregiver support resources. She did not learn anything about herself. She was sad to end the weekly sessions.” (FCG003 – 62yo White Female) |
| “She thought the time spent together has been enjoyable to talk with someone about these things.“ (PT004 - 53yo White Female, FEV1 16% - very severe COPD) “She definitely feels that she will use this tool to make decisions.” (FCG004 – 73yo White Female) |
| “Time spent together has been a great experience. Things specifically helpful included: Learning more about her health. We could do better to tell people to talk about COPD early. Learned about herself that she’s still a work in progress and learning to pay closer attention to what is going on with her health-wise and family. She feels good about ending the weekly sessions. She feels like the things we say we are going to cover gets done, and she has a better understanding.” (PT005 - 49yo Black Female, FEV1 70% - moderate COPD) |
|
“He looks forward to our calls. The living will is important. He didn't know how important it was.” (PT006 - 56yo White Male, FEV1 13% - very severe COPD) “She found our time together ‘awesome’. She found the way I helped her to be stronger and find control over things. She didn’t think of anything to make the program better. She felt that she could be herself and say what she feels, take control of what she does, how she does it, and if she wants to do it. She learned what things are ahead and hates ending our weekly sessions.” (FCG006 – 53yo White Female) |
| “She has felt ‘wonderful’ about our time together. Things she found especially helpful were the problem-solving exercise, talking about thing from past, all of it. She did not have any criticism and said the program has benefited her greatly. Regarding things she learned new about herself, she learned that ‘she needs work’. She says she is going to miss our weekly phone calls.” (PT010: 71yo Black Female, FEV1 71% - moderate COPD) “She appreciates the time we have spent together. It has helped remind her of things they need to get done. No suggestions for me to improve.” (FCG010 - 56yo Black Female) |
Abbreviations: CYC = Charting Your Course; PROs = patient reported outcomes; PT = patient; FCG = Family caregiver
DISCUSSION
Project EPIC used the NIH Stage Model to engage patients, family caregivers, and clinicians across multiple phases to complete a formative-summative evaluation of the EPIC telehealth early palliative care intervention in COPD. Data regarding acceptability and feasibility supported adaptation of ENABLE to COPD and the development of the EPIC prototype. Priority COPD early palliative care needs identified in our prior work mapped well to EPIC. The EPIC prototype intervention and data collection protocol were feasibly completed by patients and their family caregivers, and post-pilot study feedback was positive. To the best of our knowledge, EPIC is the first telephonic, nurse coach-led early palliative care intervention that engages patients across COPD severity stages and their family caregivers, who are not often included in COPD research. This study provides critical data for future EPIC refinement and testing.
Significant gaps exist on early palliative care and its effectiveness in patients with COPD,2 and Project EPIC begins to fill those gaps. Few known interventions deliver proactive palliative care before advanced COPD stages or focus a specific component of the intervention specifically on family caregivers. Existing interventions involve novel interprofessional models but typically reach patients following hospitalizations or in more severe COPD stages (e.g. end-stage COPD). For example, Rocker developed a post-acute care program for patients with severe COPD [INSPIRED (Implementing a Novel and Supportive Program of Individualized Care for Patients and Families Living with REspiratory Disease) COPD Outreach Program] using certified respiratory therapists and spiritual care practitioners who engaged them following a hospitalization or emergency department visit with action plans and psychosocial support.25,26 Rocker’s program reduced hospitalizations, emergency department visits, and hospital length of stay, and was associated with a higher frequency of home deaths.25,26 Similarly, Deunk et al. conducted the PROLONG study which delivered palliative care using an inpatient specialist palliative care team for patients hospitalized for an acute exacerbation of COPD.27 In a pragmatic cluster randomized intervention trial, there was no significant impact of the intervention on quality of life, but there was an improvement in advance care planning.27 Deunk also included participants across COPD severity stages as we did; however, their intervention was directed at patients hospitalized with COPD. In contrast, EPIC is intended for clinic and home settings to proactively address early palliative care needs.
EPIC used trained palliative care nurses as coaches to reach patients telephonically and coach them through an early palliative care program. In a similar fashion, Scheerens tested preliminary acceptability and feasibility of an intervention for people with end-stage COPD using trained palliative home care nurses who visited patients at home and educated them on COPD, coping, and self-care, conducted needs assessments, and helped build COPD action plans for crises.28 A Phase II pilot randomized controlled trial found their intervention feasible and acceptable.28 Of note, it focused primarily on patients with severe to very severe COPD (GOLD III and IV Stages), whereas EPIC reached participants across COPD severity stages, including GOLD II Stage. This shift to less severe COPD in our study may help increase palliative care’s impact in this population. Care needs can be just as high in less severe stages as we have shown, and implementing palliative care principles earlier could have a better chance at positively impacting coping skills.29 Further, recruiting a range of patients with less severe COPD enhances EPIC’s dissemination and implementation potential.
Similar to EPIC, Lindell developed a nurse-led early palliative care intervention for people living with idiopathic pulmonary fibrosis called SUPPORT (Symptom Management, Understanding the Disease, Pulmonary Rehabilitation, Palliative Care, Oxygen Therapy, Research Considerations and Transplantation).30 Trained nurses in SUPPORT delivered elements of early palliative care similar to EPIC, including illness education, symptom self-management, family caregiver support, and advance care planning. In a randomized controlled trial, SUPPORT was acceptable and demonstrated improvement in disease knowledge, further supporting the nurse-led delivery model of early palliative care.30
To promote implementation and dissemination, EPIC harnesses a low-tech telephone option that does not rely on broadband or computer availability. In ENABLE demonstration research, a telephone model was feasible in reaching rural and older adults with advanced cancer.31,32 Furthermore, the phases of Project EPIC presented here were completed just prior to the COVID-19 pandemic, which revealed a shift from reluctance in using telehealth to acceptance but also unearthed potential telehealth barriers such as limited broadband access in rural areas and issues that older adults could have with video telehealth.33,34 Notably, patient and family caregiver participants in our study viewed telehealth palliative care favorably, suggesting they supported it even before it became more acceptable during the pandemic.35 One concern we had was that despite having nurse coaches help to schedule visits in our palliative and supportive care clinic, participants experienced a delay of on average over two months to their visit, which may reflect U.S. trends in accessing ambulatory palliative care and is worthy of further exploration.1
An additional advantage to EPIC is that our intervention has a family caregiver-facing component and a nurse coach who engages them separately from their loved one. There are few, if any, COPD interventions that focus specifically on family caregivers.36,37 In our study, family caregivers feasibly completed EPIC components and also found their time with nurse coaches supportive and informative (Table 5). Our results illustrate that family caregiver participants have poor quality of life across multiple domains, which supports the importance of EPIC and offers a promising strategy to support them in future trials.
Our study was by design single-center and a small cohort. However, the project included a group of stakeholders across race, sex, and COPD severity stages and was strengthened by its multiphase design to inform intervention development, with iterative intervention refinement ongoing. We recognize a limitation in the lack of clinician diversity, which reflected the local sample of clinicians at our institution. We are enriching diversity in our ongoing intervention development research by purposively sampling a more diverse clinician population and broadening to other clinician specialties.
CONCLUSION
Project EPIC successfully adapted and pilot tested the first telephonic early palliative care intervention for patients with moderate to very severe COPD and their family caregivers. The EPIC prototype mapped well to priority COPD early palliative care needs, was feasible, and was viewed favorably by patients and their family caregivers. Project EPIC is continuing to follow the NIH Stage Model, with the prototype EPIC intervention undergoing further refinement for older adults and larger scale feasibility testing.
Supplementary Material
KEY MESSAGE.
This formative and summative evaluation study adapted and piloted tested the EPIC telephonic, nurse coach-led early palliative care intervention in adults with moderate-very severe COPD and their family caregivers. The EPIC prototype was feasible and viewed positively by participants. These findings inform future refinement and testing.
ACKNOWLEDGEMENTS
We would like to thank Sheri Tims and Elizabeth Sockwell for their diligent work as nurse coaches in this study. Without their hard work, Project EPIC would not have been possible.
DISCLOSURES
ASI reports grant support from the NIA (K76 AG064327), AHRQ (K12 HS023009), the UAB Center for Palliative and Supportive Care, and the UAB Center for Outcomes and Effectiveness Research and Education, consulting fees from Astra Zeneca, and speaking fees from Ascension St. Vincent’s.
MTD reports grant support from the American Lung Association, Department of Defense, and NIH and consulting fees from AstraZeneca, GlaxoSmithKline, Novartis, Pulmonx, and Teva. RDW, JDO, STC, EDS, LOH, AB, MA, JYB, JCD, RT, CJB, and MAB have no conflicts to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Morrison RS, Rogers M, Silvers A, Sinclair S, Heitner R, Meier DE. America’s Care of Serious Illness: 2019 State-by-State Report Card on Access to Palliative Care in Our Nation’s Hospitals. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maddocks M, Lovell N, Booth S, Man WD, Higginson IJ. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet. Sep 02 2017;390(10098):988–1002. doi: 10.1016/S0140-6736(17)32127-X [DOI] [PubMed] [Google Scholar]
- 3.Iyer AS, Sullivan DR, Lindell KO, Reinke LF. The Role of Palliative Care in COPD. Chest. May 2022;161(5):1250–1262. doi: 10.1016/j.chest.2021.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rush B, Hertz P, Bond A, McDermid R, Celi LA. Utilization of palliative care in patients with end-stage chronic obstructive pulmonary disease on home oxygen: national trends and barriers to care in the United States. Chest. Jul 4 2016;doi: 10.1016/j.chest.2016.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of End-of-Life Care Provided to Patients With Different Serious Illnesses. JAMA Intern Med. Jun 2016;doi: 10.1001/jamainternmed.2016.1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan DR, Iyer AS, Enguidanos S, et al. Palliative Care Early in the Care Continuum among Patients with Serious Respiratory Illness: An Official ATS/AAHPM/HPNA/SWHPN Policy Statement. Am J Respir Crit Care Med. Sep 15 2022;206(6):e44–e69. doi: 10.1164/rccm.202207-1262ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a Palliative Care Intervention on Clinical Outcomes in Patients with Advanced Cancer: The Project ENABLE II Randomized Controlled Trial. JAMA. Aug 19 2009;302(7):741–9. doi: 10.1001/jama.2009.1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakitas M, Lyons KD, Hegel MT, et al. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions. Palliat Support Care. Mar 2009;7(1):75–86. doi: 10.1017/S1478951509000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosemann T, Laux G, Szecsenyi J, Grol R. The Chronic Care Model: congruency and predictors among primary care patients with osteoarthritis. Qual Saf Health Care. Dec 2008;17(6):442–6. doi: 10.1136/qshc.2007.022822 [DOI] [PubMed] [Google Scholar]
- 10.Dionne-Odom JN, Azuero A, Lyons KD, et al. Family Caregiver Depressive Symptom and Grief Outcomes from the ENABLE III Randomized Controlled Trial. J Pain Symptom Manage. Jun 2 2016;doi: 10.1016/j.jpainsymman.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dionne-Odom JN, Kono A, Frost J, et al. Translating and testing the ENABLE: CHF-PC concurrent palliative care model for older adults with heart failure and their family caregivers. J Palliat Med. Sep 2014;17(9):995–1004. doi: 10.1089/jpm.2013.0680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyer AS, Dionne-Odom JN, Ford SM, et al. A Formative Evaluation of Patient and Family Caregiver Perspectives on Early Palliative Care in COPD across Disease Severity. Annals of the American Thoracic Society. Apr 30 2019;doi: 10.1513/AnnalsATS.201902-112OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakitas MA, Dionne-Odom JN, Ejem DB, et al. Effect of an Early Palliative Care Telehealth Intervention vs Usual Care on Patients With Heart Failure: The ENABLE CHF-PC Randomized Clinical Trial. JAMA Intern Med. Sep 1 2020;180(9):1203–1213. doi: 10.1001/jamainternmed.2020.2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dionne-Odom JN, Ejem DB, Wells R, et al. Effects of a Telehealth Early Palliative Care Intervention for Family Caregivers of Persons With Advanced Heart Failure: The ENABLE CHF-PC Randomized Clinical Trial. JAMA Netw Open. Apr 1 2020;3(4):e202583. doi: 10.1001/jamanetworkopen.2020.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dionne-Odom JN, Azuero A, Lyons KD, et al. Benefits of Early Versus Delayed Palliative Care to Informal Family Caregivers of Patients With Advanced Cancer: Outcomes From the ENABLE III Randomized Controlled Trial. J Clin Oncol. May 1 2015;33(13):1446–52. doi: 10.1200/JCO.2014.58.7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abedini NC, Merel SE, Hicks KG, et al. Applying Human-Centered Design to Refinement of the Jumpstart Guide, a Clinician- and Patient-Facing Goals-of-Care Discussion Priming Tool. J Pain Symptom Manage. Dec 2021;62(6):1283–1288. doi: 10.1016/j.jpainsymman.2021.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erwin K, Fitzpatrick V, Norell S, Gilliam M. Development of a Framework and Tool to Facilitate Cost-of-Care Conversations With Patients During Prenatal Care. Ann Intern Med. 2019;170(S62-S69)doi: 10.7326/M18-2207 [DOI] [PubMed] [Google Scholar]
- 18.Iyer AS, Dionne-Odom JN, Khateeb DM, et al. A Qualitative Study of Pulmonary and Palliative Care Clinician Perspectives on Early Palliative Care in Chronic Obstructive Pulmonary Disease. J Palliat Med. Apr 2020;23(4):513–526. doi: 10.1089/jpm.2019.0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMillan SC, Small BJ, Haley WE, Zambroski C, Buck HG. The COPE Intervention for Caregivers of Patients with Heart Failure: An Adapted Intervention. J Hosp Palliat Nurs. Jun 1 2013;15(4)doi: 10.1097/NJH.0b013e31827777fb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinhauser KE, Alexander S, Olsen MK, et al. Addressing Patient Emotional and Existential Needs During Serious Illness: Results of the Outlook Randomized Controlled Trial. J Pain Symptom Manage. Dec 2017;54(6):898–908. doi: 10.1016/j.jpainsymman.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 21.Weingaertner V, Scheve C, Gerdes V, et al. Breathlessness, functional status, distress, and palliative care needs over time in patients with advanced chronic obstructive pulmonary disease or lung cancer: a cohort study. J Pain Symptom Manage. Oct 2014;48(4):569–81 e1. doi: 10.1016/j.jpainsymman.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 22.Graham-Wisener L, Dempster M, Sadler A, McCann L, McCorry NK. Validation of the Distress Thermometer in patients with advanced cancer receiving specialist palliative care in a hospice setting. Palliative medicine. Sep 11 2020:269216320954339. doi: 10.1177/0269216320954339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrell BR, Twaddle ML, Melnick A, Meier DE. National Consensus Project Clinical Practice Guidelines for Quality Palliative Care Guidelines, 4th Edition. J Palliat Med. Sep 4 2018;doi: 10.1089/jpm.2018.0431 [DOI] [PubMed] [Google Scholar]
- 24.Wells R, Stockdill ML, Dionne-Odom JN, et al. Educate, Nurture, Advise, Before Life Ends Comprehensive Heartcare for Patients and Caregivers (ENABLE CHF-PC): study protocol for a randomized controlled trial. Trials. Aug 6 2018;19(1):422. doi: 10.1186/s13063-018-2770-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocker GM, Cook D. 'INSPIRED' approaches to better care for patients with advanced COPD. Clin Invest Med. Jun 01 2013;36(3):E114–20. [DOI] [PubMed] [Google Scholar]
- 26.Rocker GM, Verma JY. 'INSPIRED' COPD Outreach Program: doing the right things right. Clin Invest Med. Oct 4 2014;37(5):E311–9. [PubMed] [Google Scholar]
- 27.Duenk RG, Verhagen C, Bronkhorst EM, et al. Proactive palliative care for patients with COPD (PROLONG): a pragmatic cluster controlled trial. Int J Chron Obstruct Pulmon Dis. 2017;12:2795–2806. doi: 10.2147/COPD.S141974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheerens C, Pype P, Van Cauwenberg J, et al. Early-Integrated Palliative home care and standard care for end-stage COPD (EPIC): A Phase II pilot RCT testing feasibility, acceptability and effectiveness. J Pain Symptom Manage. Oct 9 2019;doi: 10.1016/j.jpainsymman.2019.09.012 [DOI] [PubMed] [Google Scholar]
- 29.Brien SB, Lewith GT, Thomas M. Patient coping strategies in COPD across disease severity and quality of life: a qualitative study. NPJ primary care respiratory medicine. Sep 15 2016;26:16051. doi: 10.1038/npjpcrm.2016.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindell KO, Klein SJ, Veatch MS, et al. Nurse-Led Palliative Care Clinical Trial Improves Knowledge and Preparedness in Caregivers of Patients with Idiopathic Pulmonary Fibrosis. Annals of the American Thoracic Society. Nov 2021;18(11):1811–1821. doi: 10.1513/AnnalsATS.202012-1494OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akyar I, Dionne-Odom JN, Bakitas MA. Using Patients and Their Caregivers Feedback to Develop ENABLE CHF-PC: An Early Palliative Care Intervention for Advanced Heart Failure. J Palliat Care. Apr 2019;34(2):103–110. doi: 10.1177/0825859718785231 [DOI] [PubMed] [Google Scholar]
- 32.Bakitas M, Dionne-Odom JN, Pamboukian SV, et al. Engaging patients and families to create a feasible clinical trial integrating palliative and heart failure care: results of the ENABLE CHF-PC pilot clinical trial. BMC Palliat Care. Aug 31 2017;16(1):45. doi: 10.1186/s12904-017-0226-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palma A, Rojas V, Ihl F, et al. Implementation of a Palliative Hospital-Centered Spiritual and Psychological Telehealth System During COVID-19 Pandemic. J Pain Symptom Manage. Nov 2021;62(5):1015–1019. doi: 10.1016/j.jpainsymman.2021.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dionne-Odom JN, Wells RD, Guastaferro K, et al. An Early Palliative Care Telehealth Coaching Intervention to Enhance Advanced Cancer Family Caregivers' Decision Support Skills: The CASCADE Pilot Factorial Trial. J Pain Symptom Manage. Jan 2022;63(1):11–22. doi: 10.1016/j.jpainsymman.2021.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steindal SA, Nes AAG, Godskesen TE, et al. Patients' Experiences of Telehealth in Palliative Home Care: Scoping Review. J Med Internet Res. May 5 2020;22(5):e16218. doi: 10.2196/16218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blumenthal JA, Keefe FJ, Babyak MA, et al. Caregiver-assisted coping skills training for patients with COPD: background, design, and methodological issues for the INSPIRE-II study. Clin Trials. Apr 2009;6(2):172–84. doi: 10.1177/1740774509102565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vagharseyyedin SA, Arbabi M, Rahimi H, Moghaddam SGM. Effects of a Caregiver Educational Program on Interactions between Family Caregivers and Patients with Advanced COPD. Home Healthc Now Mar-Apr 01 2022;40(2):75–81. doi: 10.1097/NHH.0000000000001049 [DOI] [PubMed] [Google Scholar]
- 38.Puhan MA, Guyatt GH, Goldstein R, et al. Relative responsiveness of the Chronic Respiratory Questionnaire, St. Georges Respiratory Questionnaire and four other health-related quality of life instruments for patients with chronic lung disease. Respiratory medicine. Feb 2007;101(2):308–16. doi: 10.1016/j.rmed.2006.04.023 [DOI] [PubMed] [Google Scholar]
- 39.Schmittdiel J, Mosen DM, Glasgow RE, Hibbard J, Remmers C, Bellows J. Patient Assessment of Chronic Illness Care (PACIC) and improved patient-centered outcomes for chronic conditions. J Gen Intern Med. Jan 2008;23(1):77–80. doi: 10.1007/s11606-007-0452-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. Sep 2009;18(7):873–80. doi: 10.1007/s11136-009-9496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment. Sep 2011;18(3):263–83. doi: 10.1177/1073191111411667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iyer AS, Wells JM, Bhatt SP, et al. Life-Space mobility and clinical outcomes in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2731–2738. doi: 10.2147/COPD.S170887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Maria M, Ferro F, Ausili D, Buck HG, Vellone E, Matarese M. Characteristics of dyadic care types among patients living with multiple chronic conditions and their informal caregivers. J Adv Nurs. Dec 2021;77(12):4768–4781. doi: 10.1111/jan.15033 [DOI] [PubMed] [Google Scholar]
- 44.Hunsley J, Best M, Lefebvre M, Vito D. The Seven-Item Short Form of the Dyadic Adjustment Scale: Further Evidence for Construct Validity. The American Journal of Family Therapy. 2001;29(4):324–335. doi: 10.1080/01926180126501 [DOI] [Google Scholar]
- 45.Montgomery RV, Stull DE, Borgatta EF. Measurement and the analysis of burden. Res Aging. Mar 1985;7(1):137–52. doi: 10.1177/0164027585007001007 [DOI] [PubMed] [Google Scholar]
- 46.Miller B, McFall S, Montgomery A. The impact of elder health, caregiver involvement, and global stress on two dimensions of caregiver burden. J Gerontol. Jan 1991;46(1):S9–19. [DOI] [PubMed] [Google Scholar]
- 47.Smaling HJ, Joling KJ, Achterberg WP, Francke AL, van der Steen JT. Measuring positive caregiving experiences in family caregivers of nursing home residents: A comparison of the Positive Experiences Scale, Gain in Alzheimer Care INstrument, and Positive Aspects of Caregiving questionnaire. Geriatr Gerontol Int. Aug 2021;21(8):636–643. doi: 10.1111/ggi.14210 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.