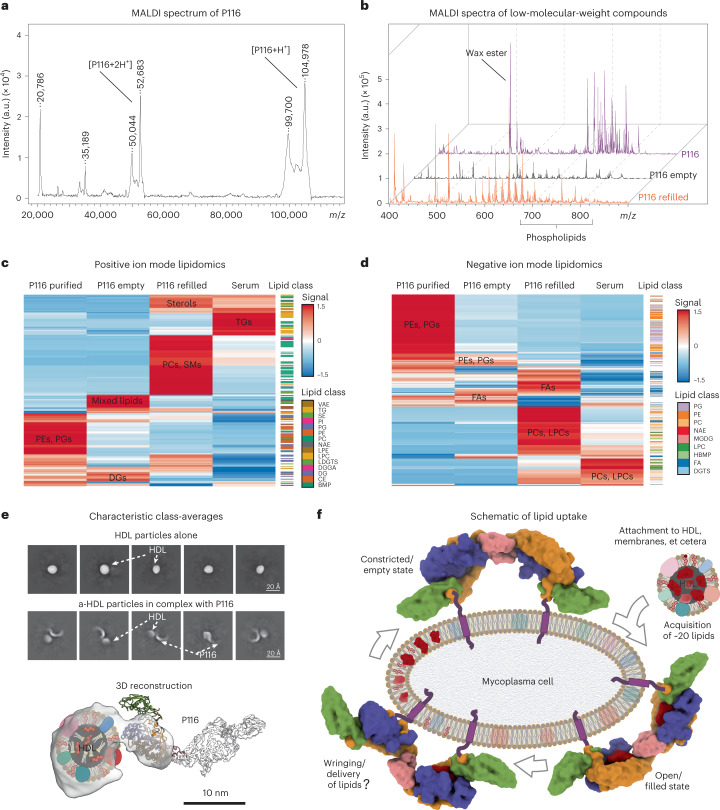
Fig. 4. Analysis of the lipid spectrum and uptake of P116.
a, MALDI-TOF mass spectrum of original P116 sample (linear mode, high mass range), showing a dominant peak at 105 kDa, corresponding to the singly charged full protein, as well as the charged states two, three and four. a.u., arbitrary units. b, Stacked MALDI-TOF mass spectra (reflector mode, low mass range) of the original purified P116 (purple, rear), empty P116 (black, middle) and refilled P116 sample (orange, front) showing a change in the lipid distribution among the samples. c,d, Hierarchical clustering of lipid compounds identified in positive (c) and negative (d) ion mode lipidomics (LC–MS/MS) analyses (reproduced in three independent experiments), showing differential distributions of lipid compositions in original P116 (first column), empty P116 (second column), refilled P116 (third column) and serum (fourth column). The refilled P116 shows a particular affinity to sterols and cholesterol specifically. All data were normalized to the mTIC of all identified compounds in each sample, and row-wise scaling was applied. PE, phosphatidylethanolamine; PG, phosphatidylglycerol; DG, diacylglycerol; PC, phosphatidylcholine; SM, sphingomyelin; TG, triacylglycerol; FA, fatty acid; LPC, lysophosphatidylcholine; VAE, vitamin A fatty acid ester; SE, sterol esters; PI, phosphatidylinositol; NAE, N-acyl ethanolamines; LPE, lysophosphatidylethanolamine; LDGTS, lysodiacylglyceryl trimethylhomoserine; DGGA, diacylglyceryl glucuronide; CE, cholesteryl ester; BMP, bismonoacylglycerophosphate; NAE, N-acyl ethanolamines; MGDG, monogalactosyldiacylglycerol; HBMP, hemibismonoacylglycerophosphate; DGTS, diacylglyceryl trimethylhomoserine. e, CryoEM analysis of empty P116 incubated with HDL shows that P116 binds HDLs between its N-terminal and core domains. P116 is attached to HDL through its distal core. Owing to the flexibility of P116 and the variability of HDL, only one subunit of P116 can be seen at this threshold. The whole P116 can be seen in the individual class averages. f, Schematic of the lipid uptake and conformational variations of P116 (here indicated by its structure anchored in the mycoplasma membrane. Linkers and transmembrane domains not seen in the cryoEM structure are shown in purple). P116 starts in an empty, constricted state; incubation with HDL leads to each individual monomer filling up with approximately 20 lipids; and P116 changes to the open/filled state. We hypothesize that, through a wringing motion, lipids are delivered into the mycoplasma membrane.