Fig. 3.
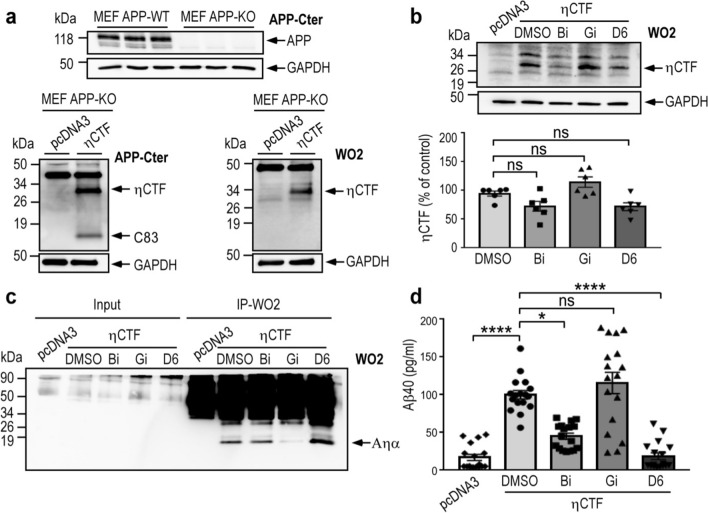
ηCTF fragment yields both Aηα and Aβ peptides. a Wild-type (MEF APPwt) and APP/APLPs-deficient mouse embryonic fibroblasts (MEF APPKO) were transiently transfected with ηCTF or pcDNA3 vectors and analyzed by western blot using APP-Cter and WO2 antibodies. GAPDH is used as loading control. b–d ηCTF transfected MEF APPKO cells were treated for 24 h with α- β- or γ-secretase inhibitors (Gi:10 µM, Bi:30 µM, D6:1 µM) then analyzed by western blot using WO2 antibody (b). GAPDH is used as loading control. Bars correspond to the quantification of ηCTF immunoreactivity expressed as percent of controls (DMSO-treated cells) taken as 100 and are the means ± SEM of 6 independent determinations. Ns, not statistically significant according to the Tukey one-way ANOVA test (b). Aηα peptides were immunoprecipitated (IP) using WO2 antibody from conditioned medium of MEF APP KO cells expressing or not ηCTF and treated with α- β- or γ-secretase inhibitors. Note that Aηα was not detectable in secretates before immunoprecipitation (Input) (c). Aβ40 levels were measured by ELISA in the conditioned medium of MEF APPKO cells expressing or not ηCTF and treated with α- β- or γ-secretase inhibitors. Bars indicate the concentration of Aβ in pg/ml and are the means ± SEM of 17 independent determinations. ****p < 0.0001, *p < 0.05, ns: not statistically significant according to the Tukey one-way ANOVA test (d). All full gels are provided in Sup Fig. 5