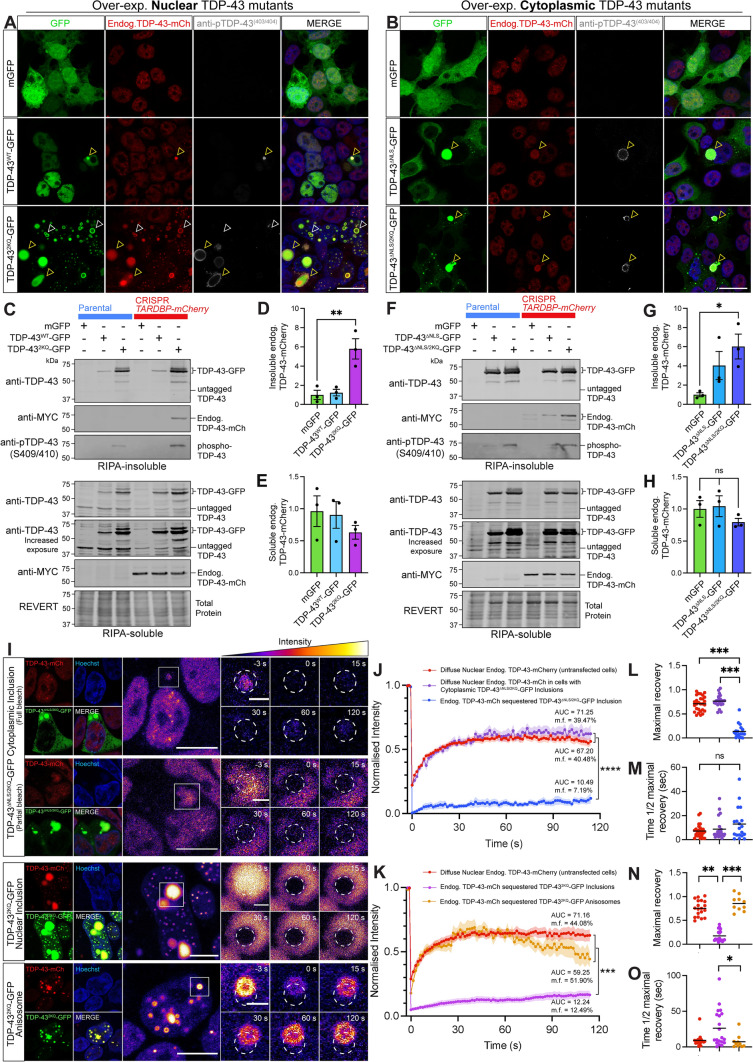
Fig. 6.
Sequestered endogenous TDP-43 becomes insoluble and immobile in nuclear and cytoplasmic inclusions, however retains high protein mobility when recruited to nuclear anisosomes. a, b Representative confocal images of CRISPR TARDBP-mCherry cells expressing a nuclear or b cytoplasmic wild-type or acetylation-mimicking mutant TDP-43-GFP, or mGFP control, 48 h post-transfection. Yellow arrowheads indicate TDP-43-GFP inclusions comprising endogenous TDP-43-mCherry and immunostained with phosphorylated TDP-43. White arrowheads indicate acetylated TDP-43 anisosomes which sequestered endogenous TDP-43-mCherry but lacked phosphorylated TDP-43 (scale = 20 µm). c, f Immunoblotting of CRISPR TARDBP-mCherry cells expressing c nuclear or f cytoplasmic mutants demonstrates changes in endogenous TDP-43-mCherry levels within d, g RIPA-insoluble and e, h soluble protein fractions, respectively, quantified by densitometry analysis. i FRAP was used to quantify the mobility of endogenous TDP-43-mCherry proteins recruited to distinct nuclear or cytoplasmic structures formed by the over-expression of acetylation-mimic TDP-43-GFP mutants. Scale bars on left-hand panels = 10 µm; zoomed right-hand panels = 2 µm. Pseudocolouring demonstrates changes in endogenous TDP-43-mCherry intensity, which was quantified within the bleaching region-of-interest (white dotted circle) for 120 s post-bleaching. FRAP analysis of the normalised intensity during the recovery period for cells expressing j cytoplasmic or k nuclear acetylation-mimic mutant TDP-43-GFP, along with mobility fraction (m.f.) values and area-under-the-curve (AUC) averaged for each cell, which was used for statistical comparison. l, n Maximal fluorescence intensity observed at the final time-point (120 s), and m, o time taken to recover to half of the maximal fluorescence level for nuclear and cytoplasmic TDP-43 mutants respectively. Same colour scheme as the intensity line graphs, with each point representing a single cell value, and the black line representing the mean across n = 3 biologically independent repeats. Statistically significant differences between the means were analysed using one-way ANOVA multiple comparisons with Tukey’s post hoc test (*P < 0.05; **P < 0.01)