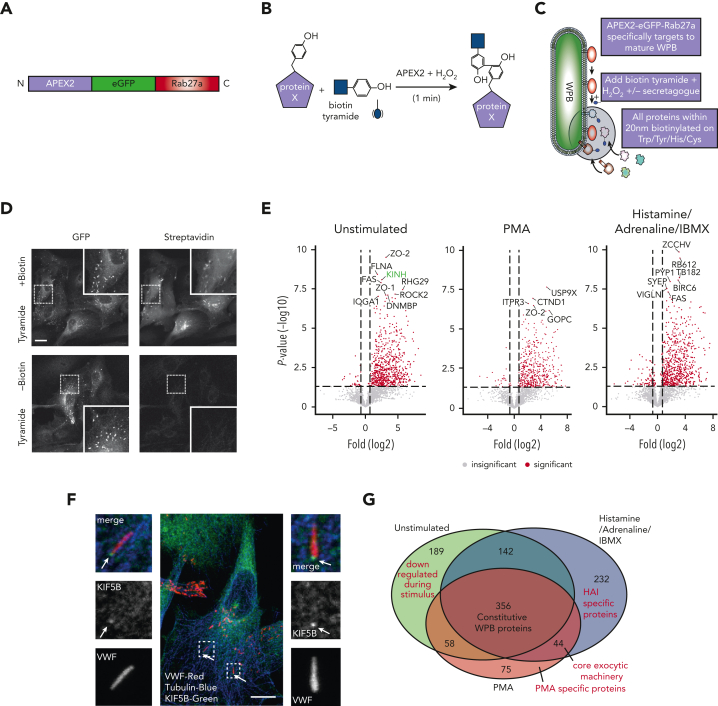
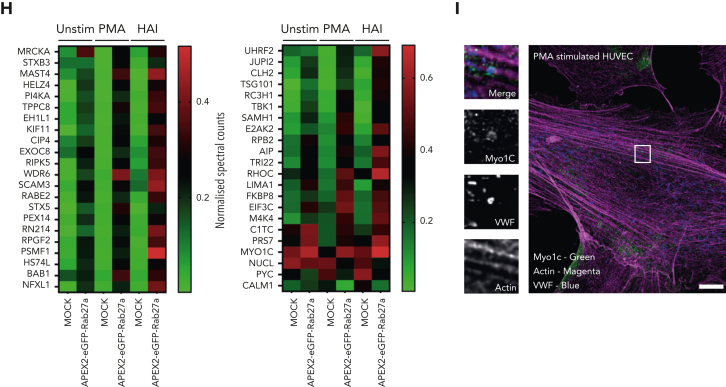
Figure 1.
Proximity proteomics reveals novel WPB-associated machinery. (A) Schematic representation of the APEX2-eGFP-Rab27a construct used to transfect HUVECs. (B) Biotinylation reaction catalyzed following the addition of hydrogen peroxide and biotin tyramide. (C) The APEX2-eGFP-Rab27a construct is recruited to mature WPBs, allowing spatiotemporally-specific biotin labeling on nearby proteins. The addition of stimulants allows detection of proteins with putative roles in basal and regulated granule release. (D) Confocal LSM of HUVECs in the presence or absence of biotin tyramide. Biotinylated proteins are detected using AF647-conjugated streptavidin and colocalized with the enhanced green fluorescent protein (eGFP)−tagged fusion protein. (E) Scatter graphs depicting the most significant (paired t test) and upregulated proteins as compared to mock-transfected control HUVECs in unstimulated, PMA-stimulated, and HAI-stimulated cells. Kinesin-1 heavy chain (KINH) (green) was used to validate the data set. (F) IF analyses of HUVECs illustrate KIF5B (KINH) localizing to one end of WPBs under resting conditions. Scale bar, 10 μm. (G) Venn diagram of mass spectrometry data sets illustrates the number of proteins in close proximity to WPBs under all conditions, exclusively under basal conditions or following stimulation. (H) Heat map depiction of the average normalized spectral counts of the 44 stimulation-associated proteins in mock and APEX2-eGFP-Rab27a groups (in the presence or absence of PMA/HAI). (I) Myosin-1c (green) is present at points of WPB fusion in PMA-stimulated HUVECs. Scale bar, 10 μm.