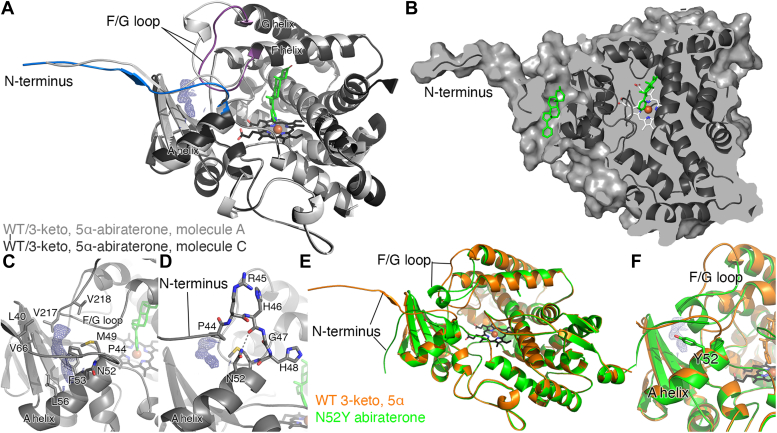
Figure 4.
A peripheral ligand binding site in the CYP17A1 WT structure with the 3-keto, 5α abiraterone analog and comparison to the CYP17A1 N52Y mutant structure with abiraterone.A, overlay of the CYP17A1/3-keto, 5α-abiraterone conformations for molecule A (light grey) and molecule C (dark gray with blue N-terminus and purple F/G loop) highlights their conformational similarity except for these two regions. The peripheral ligand density (blue mesh) is only present in the conformation represented in molecules C and D and is flanked by the beta sheet system, the F/G loop, the N-terminus, and a portion of the A helix. B, surface view of the same CYP17A1 structure (dark gray, molecule C), using a slightly different rotation and cutaway to show the cavity for the peripheral ligand (green sticks) and its relationship to the copy in the active site (green sticks). C, detailed view of the peripheral binding site in the 3-keto, 5α abiraterone analog shows surrounding residues but does not indicate specific interactions with the ligand. D, in rationalizing a mutant to block occupation of the peripheral site, N52 appeared to be a good candidate since the favorable rotamer if mutated to a tyrosine would project into the peripheral binding site. E, comparison of the CYP17A1 WT cocrystallized with 3-keto, 5α abiraterone structure (chain C, orange) and the structure of the CYP17A1 N52Y mutant cocrystallized with abiraterone (chain B, green) reveals that the overall conformation is maintained, but differences in conformation are observed in the N-terminus and F/G loop. In all molecules, the N52Y mutant adopts a unique conformation for both of these elements, and electron density is not observed in the peripheral site. F, a detailed view of the peripheral binding site illustrating how the mutated tyrosine projects into the peripheral site as designed. All maps shown are 2Fo-Fc; maps generated without ligands and contoured at 1 σ (blue mesh).