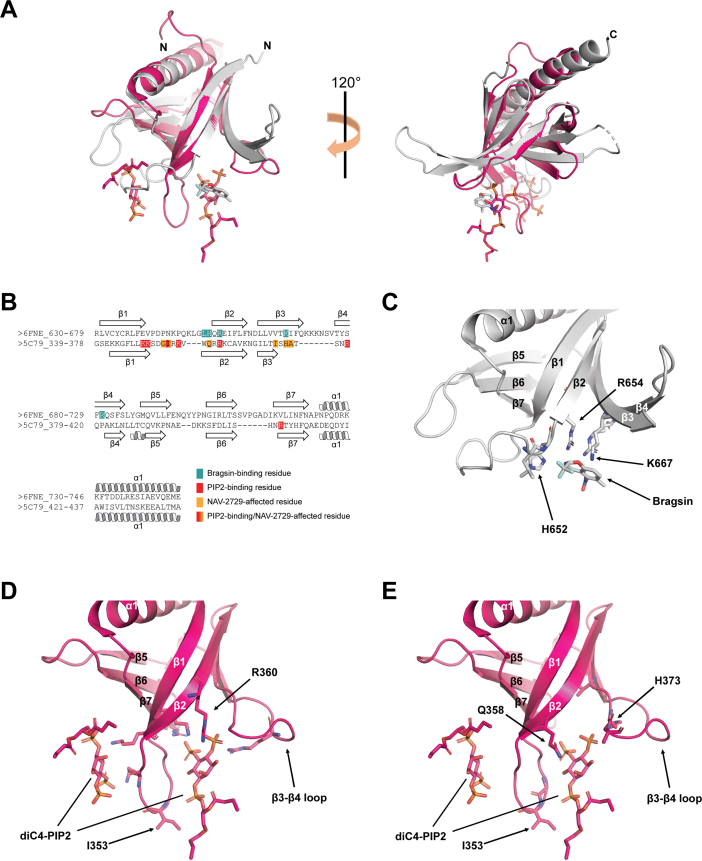
Figure 7.
Superposition and primary sequence alignment of Brag2PH:Bragsin and ASAP1PH:diC4-PIP2 structures.A, overall superposition of Brag2PH:Bragsin (gray) and ASAPPH:diC4-PIP2 (pink). Bragsin is shown as gray sticks and the two diC4-PIP2 molecules are shown as pink sticks. N- and C-termini are labeled. B, alignment of Brag2PH (6FNE_630-746) and ASAP1PH (5C79_339-437) residues based on structural superposition. Brag2PH residues that bind to Bragsin are colored teal. ASAP1PH residues that bind to PIP2 are shown in red, while those that exhibit chemical shift perturbations upon binding to nanodiscs containing NAV-2729 are shown in orange. The single ASAP1PH residue that binds to both PIP2 and exhibits chemical shift perturbations upon binding NAV-2729 nanodiscs (I353) is shown in a red/orange gradient. Secondary structure elements are labeled. C, Brag2PH residues that bind to Bragsin. R654 is labeled to indicate its positioning relative to R360 in (D). H652 and K667 are labeled to indicate their positioning relative to Q358 and H373 in (E). Secondary structure elements are labeled. D, ASAP1PH residues that bind to diC4-PIP2. R360 is labeled to indicate its positioning relative to R654 in (C). I353 is labeled as it is the sole residue that binds diC4-PIP2 and exhibited chemical shift perturbations upon binding nanodiscs containing NAV-2729. Secondary structure elements are labeled. E, ASAP1PH residues that exhibit chemical shift perturbations upon binding to nanodiscs containing NAV-2729. Q358 and H373 are labeled to indicate their positioning relative to H652 and K667 in (C). All other features are labeled as described in (D). PH, pleckstrin homology; PIP2, phosphatidylinositol 4,5-bisphosphate.