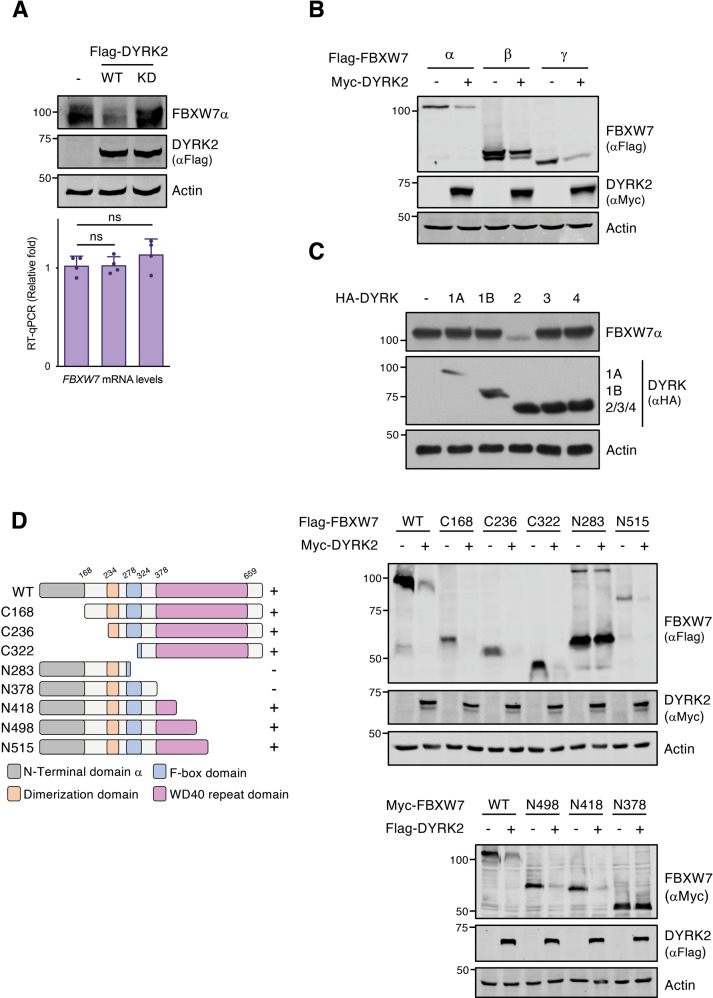
Fig. 1. FBXW7 protein levels are regulated by DYRK2.
A HEK-293T cells were transfected (2 × 105 cells/35-mm dish) with 0.4 μg of either the control expression vector, wild type (WT) or kinase-dead (KD, mutation in the ATP binding site K178M) DYRK2 expression vectors and cells harvested after 48 h. One fraction was used to analyze endogenous FBXW7α and exogenously expressed DYRK2 protein levels by immunoblotting (upper panel, a representative experiment is shown, n = 4), while another aliquot was used to analyze FBXW7α mRNA levels (lower panel, the graph shows relative RNA levels determined by RT-qPCR with mock-transfected cells set up as 1; mean ± SD, n = 4; ns, not significant). B HEK-293T cells (2 × 105 cells/35-mm dish) were transfected with plasmids to express FBXW7 isoforms α, β, and γ (0.2 μg) together with Myc-DYRK2 (0.2 μg) or the control expression vector (0.2 μg). Cells were lysed after 48 h and protein expression analyzed by WB. C Endogenous FBXW7α protein expression was evaluated by WB in HEK-293T cells expressing the five different human DYRK family members. D The indicated Flag-tagged truncated versions of FBXW7α were transiently overexpressed in the presence or absence of Myc-DYRK2 in HEK-293T cells. The schematic representation of FBXW7α indicates the positions of the N-terminal domain (grey), the dimerization domain (orange), the F-box domain (blue), and the region containing the WD40 domains (pink). Note: a representative experiment is shown in each panel of 3–4 performed.