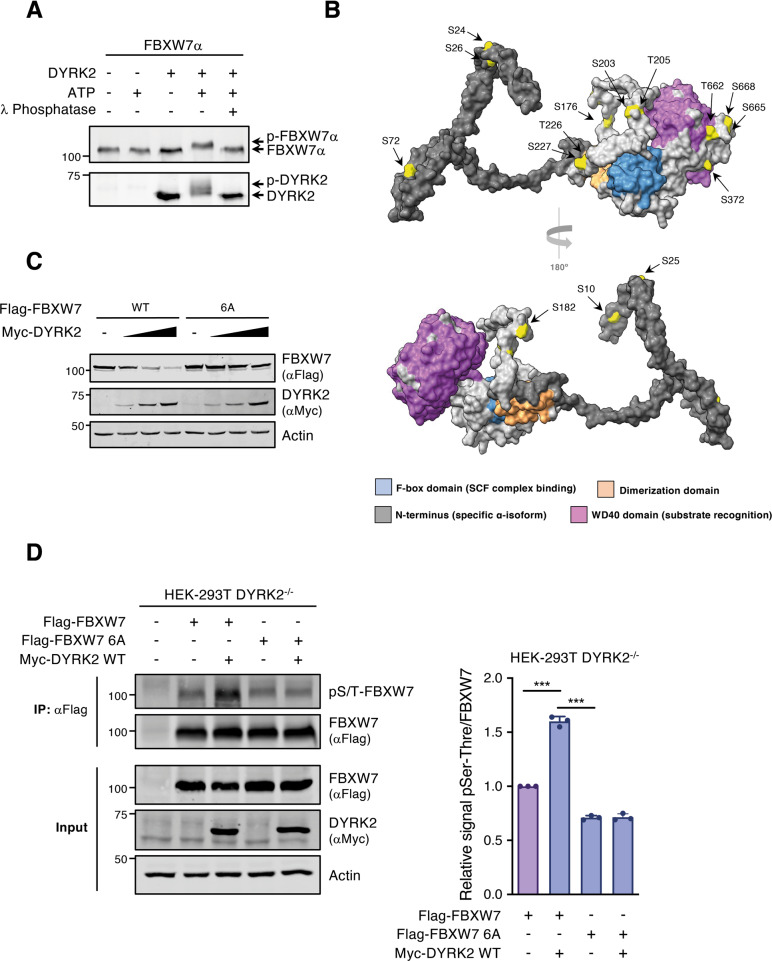
Fig. 4. DYRK2 phosphorylates FBXW7.
A FBXW7α and DYRK2 recombinant proteins were incubated in the presence of ATP and λ-phosphatase and analyzed by WB. Note the electrophoretic mobility shifts in the two proteins, which in the case of DYRK2 are due to autophosphorylation [29]. B Surface-filling models of FBXW7α 3D structure prediction shown from different angles; the color code is for the different domains and the phosphorylated amino acids identified by MS are in yellow. C Extracts from cells expressing Flag-FBXW7α WT or a mutant version with six S/T-to-A changes (6 A: S176, S182, T205, S227, S372, and S688), either alone or together with increasing amounts of Myc-DYRK2, were analyzed by WB. D HEK-293T DYRK2-KO cells were transfected with Flag-FBXW7α WT or Flag-FBXW7 6 A mutant in the presence or absence of Myc-DYRK2 as indicated, followed by anti-Flag immunoprecipitation. Cells were previously treated with MG-132 (10 μM) to avoid FBXW7 degradation. Total FBXW7α was detected with a Flag antibody and phosphorylated FBXW7α with an antibody detecting phospho-serine and phospho-threonine residues (pS/T). The bar graph shows the ratio phospho-FBXW7/total FBXW7 in the immunoprecipitates, set as 1 in the absence of Myc-DYRK2 (mean ± SD, n = 3; **P < 0.01). Note: a representative experiment is shown in each panel of 3–4 performed.