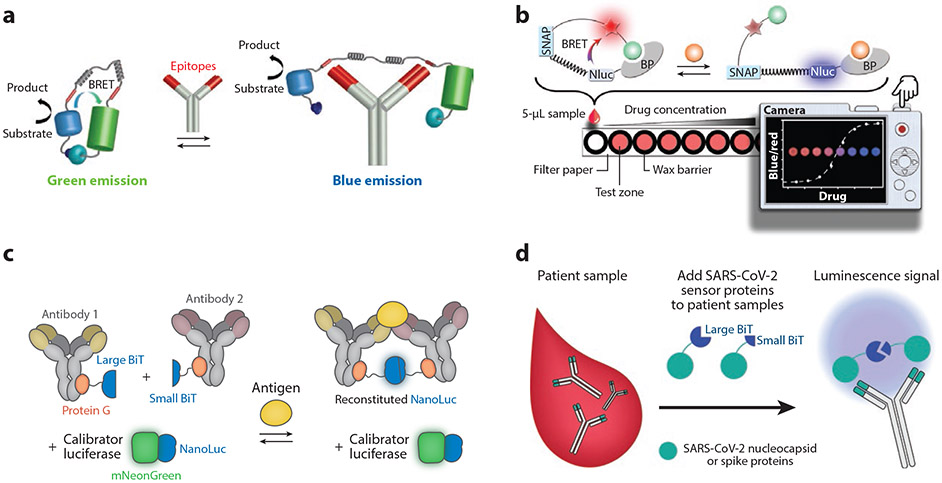
Figure 4.
Bioluminescence immunoassays. (a) Luminescent antibody sensor (LUMABS) assay. In the absence of the antibody of interest, NanoLuc and mNeonGreen are in close proximity, enabling efficient bioluminescence resonance energy transfer (BRET). Bivalent binding of the antibody of interest to the epitopes (red) in the semiflexible linker pulls the BRET partners apart, changing the color of emission from green to blue. Panel adapted with permission from Reference 74; copyright 2016 American Chemical Society. (b) A luciferase-based indicators of drugs (LUCID) sensor, composed of a SNAP-tag, NanoLuc (Nluc), and a binding protein (BP). The SNAP-tag is labeled with a molecule containing a fluorophore (red star) and a ligand (green ball) that can bind to the BP. The analyte of interest displaces the ligand, changing emission from red to blue, which is imaged by a digital camera. Panel adapted with permission from Reference 77; copyright 2017 John Wiley and Sons. (c) The ratiometric plug-and-play immunodiagnostics (RAPPID) platform. A pair of antibodies for an antigen of interest are functionalized with a split version of NanoLuc (large BiT and small BiT) via protein G–based photoconjugation. Antigen binding brings the antibody pair into proximity, resulting in the reconstitution of NanoLuc and emission of blue light after addition of the substrate. Panel adapted with permission from Reference 82 (CC BY-NC 4.0). (d) Split luciferase (spLUC) assay for anti-SARS-CoV-2 antibody detection. Antibodies are incubated with SARS-CoV-2 spike or nucleocapsid proteins fused to large BiT and small BiT. Approximately half of the antibodies will bind to the large BiT fusion with one arm and the small BiT fusion with the other, reconstituting active NanoLuc, which generates luminescence.