Abstract
Study Design.
Prospective Randomized Placebo Controlled Animal Trial
Objective.
Determine the effect of daily subcutaneous abaloparatide injection on the intervertebral fusion rate in rabbits undergoing posterolateral fusion.
Study of Background Data.
Despite the wide utilization of spine fusion, pseudarthrosis remains prevalent and results in increased morbidity. Abaloparatide is a novel analog of parathyroid hormone-related peptide (1–34) and has shown efficacy in a rat posterolateral spine fusion model to increase fusion rates. The effect of abaloparatide on the fusion rate in a larger animal model remains unknown.
Methods.
24 skeletally mature New Zealand White male rabbits underwent bilateral posterolateral spine fusion. Following surgery, the rabbits were randomized to receive either saline as control or abaloparatide subcutaneous injection daily. Specimens underwent manual assessment of fusion, radiographic analysis with both x-ray and high-resolution peripheral quantitative computed tomography, and biomechanical assessment.
Results.
Rabbits that received abaloparatide had a 100% (10/10) fusion rate compared to 45% (5/11) for controls (p<0.02) as assessed by manual palpation. Radiographic analysis determined an overall mean fusion score of 4.17 ± 1.03 in the abaloparatide group versus 3.39 ± 1.21 for controls (p< 0.001). The abaloparatide group also had a greater volume of bone formed with a BV of 1209 ± 543mm3 compared to 551 ± 152mm3 (p<0.001) for controls. The abaloparatide group had significantly greater trabecular bone volume fraction and trabecular thickness and lower specific bone surface and connectivity density in the adjacent levels when compared to controls. Abaloparatide treatment did not impact trabecular number or separation. There were no differences in biomechanical testing in flexion, extension, or lateral bending (p>0.05) between groups.
Conclusion.
Abaloparatide significantly increased the fusion rate in a rabbit posterolateral fusion model as assessed by manual palpation. Additionally, there were marked increases in the radiographic evaluation of fusion.
Keywords: Spine Fusion, Adjacent Segment, Rabbit Model, Lumbar Fusion, Treatment, Biologics, Bone Formation
Introduction
Spinal fusion, or arthrodesis, is a surgical procedure designed to stabilize the spinal column by creating an osseous bridge between adjacent vertebral segments. It is utilized in a wide variety of spinal pathologies, including degenerative disease, trauma, and reconstruction for neoplastic conditions. The utilization of spine fusion was reported at over 400,000 cases annually in 2008 and continues to increase.1–3
Along with the increasing primary spine fusion rate, the number of spine fusion revisions is rising partly due to pseudoarthrosis or non-union.4 Those who undergo revision due to a failure to achieve bony union result in increased patient morbidity, re-operation rates, and higher cost.4 To enhance the spine fusion success rate, surgical fixation techniques that augment the biomechanical stability of the fusion mass, such as interbody devices, as well as biologic adjuncts such as bone morphogenetic protein (BMP), are being utilized.4,5
Parathyroid hormone (PTH) is the primary endogenous hormone responsible for regulating bone and calcium metabolism and has a catabolic effect on bone with continuous exposure. The use of intermittent PTH 1–34 to increase spine fusion has been well studied in both rat- and rabbit- posterolateral spine fusion models.6–12 Furthermore, PTH 1–34 has been shown to enhance spine fusion rates in patients with osteoporosis.13,14
Abaloparatide is a newer investigational drug analog of human parathyroid hormone-related peptide (hPTHrP) and is a potent PTH receptor agonist. Abaloparatide is a 34 amino acid peptide with 76% homology to hPTHrP 1–34, and 41% homology to hPTH (1–34).15 In osteopenic rat models, abaloparatide increased trabecular and cortical bone, cortical thickness, trabecular bone formation, bone strength, and bone mineral density but unlike PTH(1–34), it does not increase as bone resportion.15,16 Recently, abaloparatide was also shown to markedly enhance the spine fusion rate in a rat posterolateral spine fusion model.17
The purpose of this study was to determine the effect of daily subcutaneous abaloparatide injection on the intervertebral lumbar fusion rate in rabbits. Additionally, we aimed to evaluate the effects on adjacent vertebra for bone volume increases.
Methods
Study Overview
Twenty-four skeletally mature New Zealand White (NZW) male rabbits were used in this study. All rabbits underwent bilateral lumbar posterolateral spine fusion with iliac crest bone graft. Six-month old rabbits were purchased from Envigo (Indianapolis, IN) and housed in United States Department of Agriculture (USDA) approved cages under the direct supervision of institutional veterinary staff. The study was approved by the combined Hospital for Special Surgery, Memorial Sloan Kettering, and Weill Cornell Medical College Institutional Animal Care and Use Committee (IACUC), protocol number 2017-0045.
Animals were randomly assigned to one of two treatment groups. Starting on post-operative day four and following posterolateral spine fusion surgery as described below, Group 1 (control) received daily subcutaneous saline injections while Group 2 (abaloparatide) received abaloparatide 25 μg/kg/d using a 2000 mcg/mL solution. The abaloparatide dose of 25 mcg/kg was determined by previously performed preclinical data.16,17 Abaloparatide was obtained from Radius Health (Boston, Massachusetts) as a lyophilized powder. The powder was stored at −20°C, resuspended, and then aliquoted for the daily treatment dose.
Surgical Protocol
All surgeries were performed using aseptic surgical procedures adapted for the rabbit per the model developed by Boden et al.18 The surgical protocol was approved by the IACUC. In brief, a dorsal midline skin incision was made followed by two paramedian fascial incisions. The intermuscular plane between the multifidus and longissimus muscles was developed to expose the L4 and L5 transverse processes. Through a separate dorsal fascial incision, the iliac crest was harvested subperiosteally from a single side. The iliac crest autograft was morselized and measured with an open-ended syringe by applying compression on the plunger to a volume of 1 cm3. A graft volume of 1 cm3 has been shown to produce lower fusion rates and this volume was chosen for the potential to show an additional benefit of abaloparatide administration.9,19 Using a surgical burr and/or rongeurs, the transverse processes were decorticated to expose bleeding bone. The autograft was then placed between the transverse processes. The animals were closed in standard fashion.
Immediate post-operative radiographs were obtained to confirm the fusion level. Given the variable lumbar anatomy of the rabbit, graft placement between both the L4/L5 and L5/L6 transverse processes bilaterally were considered a successful experimental surgery.
Animals were sacrificed at 42 ± 3 days postoperatively through the administration of acetyl-promazine, 1 mg/kg intra- muscularly, followed 20 minutes later by sodium pentobarbital (26% solution), 2 cc intravenous by veterinary staff. In vivo radiographs were taken immediately following euthanasia.
Manual Assessment of Fusion
The lumbar spine was removed and either the L4/L5 or L5/L6 spinal segment was removed en-bloc. The surrounding soft tissues and musculature were removed to expose the vertebrae without disruption of the fusion mass. A manual assessment of fusion of each spinal segment was performed immediately following spine harvesting to evaluate fusion similar to prior studies.7–9,18,20,21 The spinal segments were assessed in both flexion/extension and lateral bending. Three blinded raters evaluated the motion of the segment on a binary scale (0, 1) as follows: 0 – No fusion: unrestricted motion, 1: Fusion – restricted motion in all planes. Specimens were considered fused if two raters graded the specimen as “fused.”
Radiographic and Computed Tomography Assessment
Post-sacrifice radiographs were analyzed by three blinded raters using a scoring system (“0” [no bone] through “5” [definite fusion]). Raters were asked to score the fusion mass on each side and scores were averaged for both abaloparatide and control groups.
High-resolution peripheral quantitative computed tomography (HR-pQCT) scans of the fused lumbar vertebrae were performed with the Siemens Inveon PET/CT (Siemens, Munich, Germany). Scans were acquired with a step and shoot setting at 80 kV, 500 uA, 200 ms/projection, and a pixel size of 98 micron. Regions of interest (ROI) of each fusion mass were created to assess bone volume (mm3).
Furthermore, the scans were sent to a collaborator at Beth Israel Deaconess Medical Center (BIDMC) for further analysis. The goal of the analysis at BIDMC was to determine whether treatment with abaloparatide (ABL) improved vertebral trabecular bone architecture following spinal fusion surgery on adjacent vertebra. Scans that were reconstructed with a 72 μm isotropic voxel size were pre-processed at BIDMC and imported into micro-computed tomography analysis software (Scanco Medical software suite, Brüttisellen, Switzerland) for analysis. Trabecular architecture was analyzed in the endocortical region of the caudal aspect of the body of the more cranial of the two fused vertebrae, in a region beginning at an eighth of the vertebral body height superior to the distal end of the vertebral body and extending cranially 43 slices (≈3mm) (Figure 1).
Figure 1:
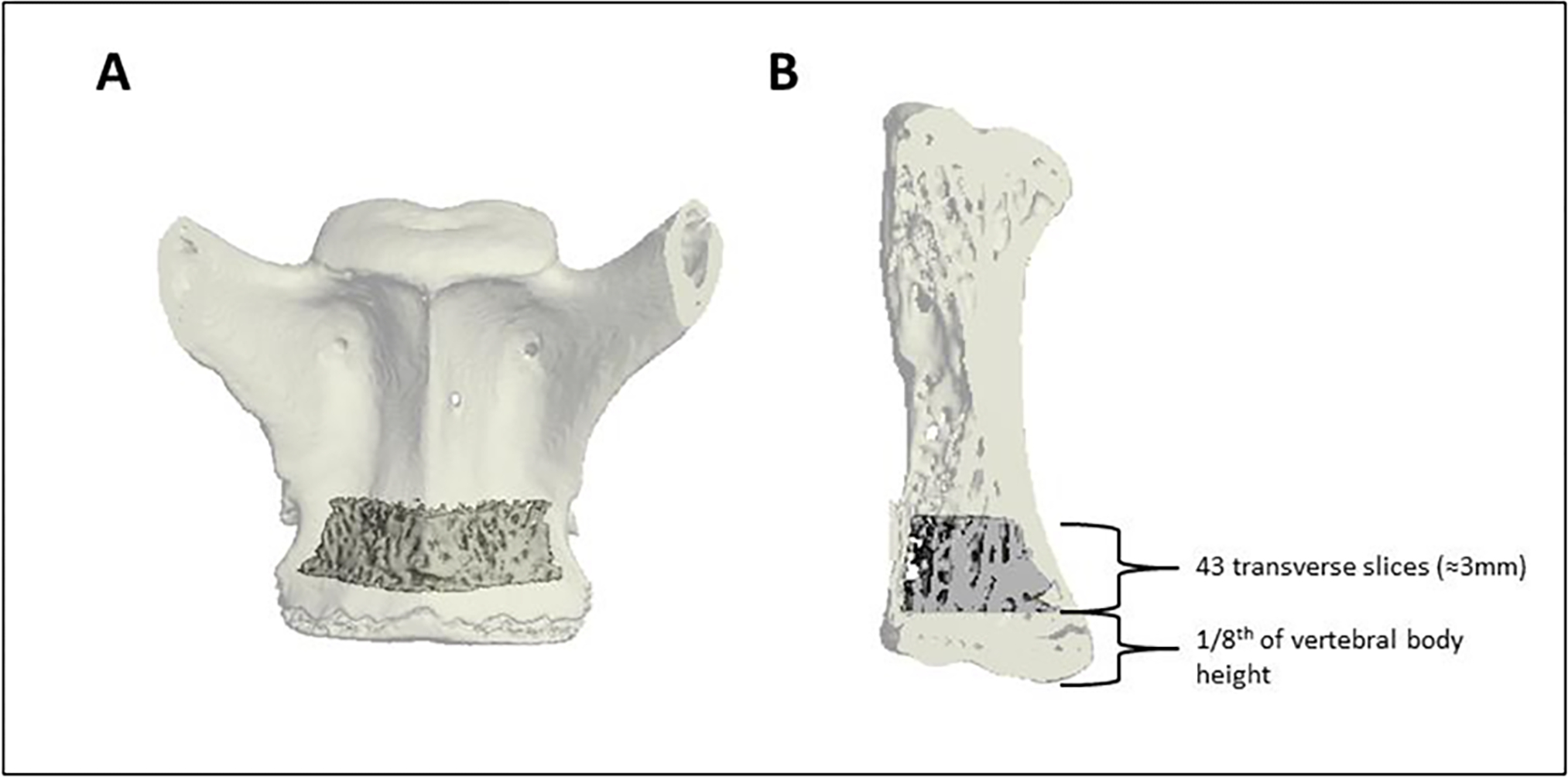
Coronal (A) and sagittal (B) views of the trabecular region of interest (ROI) in the caudal aspect of the more cranial of the two fused vertebrae. The trabecular region of interest began an eighth of the vertebral body height superior to the distal end of the vertebrae and extended cranially 43 transverse slices (≈3mm). The trabecular ROI is shown as solid within the transparent shell of the vertebrae.
Adaptive iterative thresholding (AIT) was performed to determine the segmentation threshold used to segment bone from soft tissue in each scan, and then applied the standard Scanco trabecular bone morphometry script to measure the following trabecular architectural parameters: bone volume fraction (Tb.BV/TV, %), trabecular specific bone surface (Tb.BS/BV, mm2/mm3), trabecular thickness (Tb.Th, mm), trabecular number (Tb.N, mm−1, trabecular separation (Tb.Sp, mm), and connectivity density (ConnD, 1/mm3).
Biomechanical Assessment
13 specimens were randomly assigned to undergo biomechanical testing with equal distribution amongst the groups. The study protocol was described previously by Cottrell et al.22 In brief, a four-point bending experiment was conducted using an MTS Load-frame Model 312.21 (MTS Systems Corporation, Eden Prairie, MN). The vertebrae were stored in air-tight sealed containers at −20°C. The adjacent vertebrae were potted using polymethyl methacrylate (PMMA) in aluminum tubes with transverse process cut-outs to avoid motion interference leaving only the surgical level exposed. Custom fixtures were utilized with an exterior support span of 95.2mm, with the interior span offset by 19.0mm on each side. Specimens were loaded with five load-unload cycles to 40N at a 5-N/s rate in flexion, extension, right lateral bending, and left lateral bending. The first four cycles were used to precondition the sample to remove creep, and the fifth cycle was used for analysis to determine the bending stiffness (N/m2).
Statistics
The primary outcome was the fusion rate using the manual assessment of fusion. We hypothesized that the abaloparatide group would show increased fusion rate compared to the control group. A power analysis was performed assuming that abaloparatide will have a similar fusion rate based on manual bending as the PTH analog teriparatide (81% vs. 30% saline control) in the study by O’Loughlin et al.9 Using power analyses at 0.80 beta & 0.05 alpha, a sample size of 11 rabbits per group was calculated to be needed to detect this difference.
The manual palpation data was analyzed with Fisher’s exact test. Secondary outcomes were load-displacement via biomechanical testing and bone volume based upon HR-pQCT, and t-tests were utilized to analyze biomechanical load-displacement and bone volume to total volume ratio measured by computed tomography. Intraclass correlation coefficients (ICC) were calculated to determine inter-rater reliability for all observations. All analyses were performed with SPSS version 27 (International Business Machines, Armonk, NY).
Results
A total of 24 rabbits underwent posterolateral spine fusion. Three rabbits did not survive surgical anesthesia, leaving 11 rabbits in the control group and 10 in the abaloparatide group.
Manual Assessment of Fusion (MAF)
MAF testing identified successful fusion in 45% (5/11) of control animals compared to 100% (10/10) of abaloparatide treated animals (p < .02) (Figure 2). Measurement showed good reliability with an ICC of 0.834 (95% CI 0.656–0.927) with raters having 100% agreement in 90% (9/10) of abaloparatide treated animals.
Figure 2:
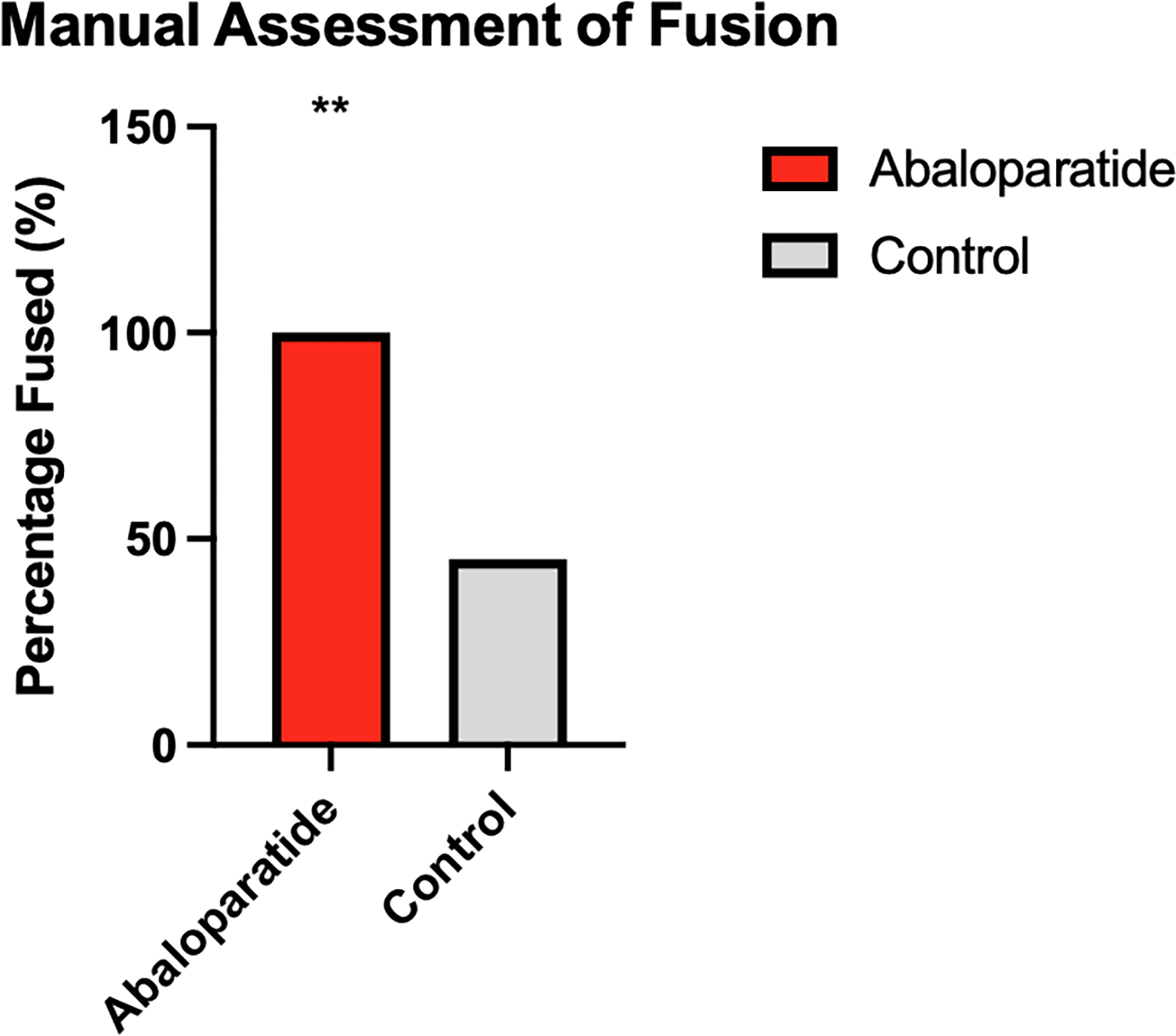
Manual Assessment of Fusion Results. ** p<0.02.
Radiographic and Computed Tomography Assessment
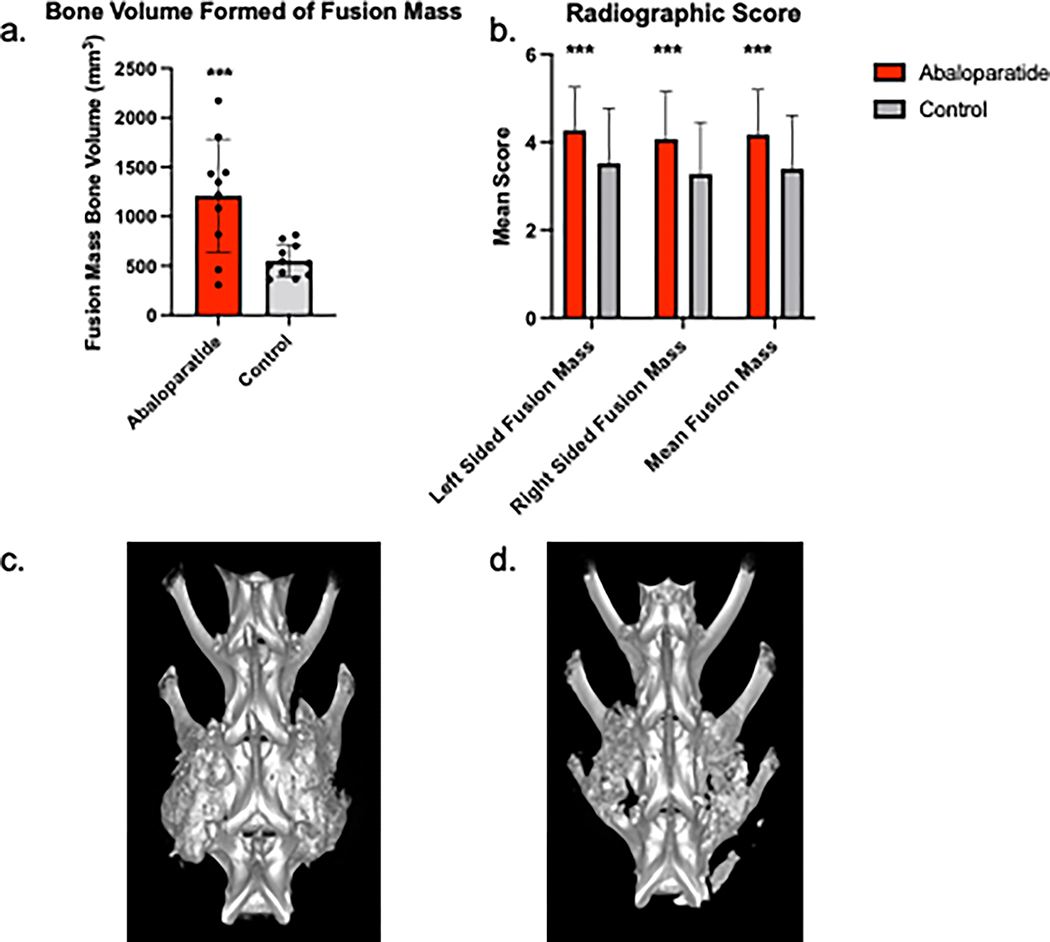
Radiographic analysis determined an overall mean fusion score of 4.17 ± 1.03 in the abaloparatide-treated animals versus 3.39 ± 1.21 in the saline control treated animals (p < .001). The left and right-sided radiographic scores were 4.26 ± 0.98 and 4.07 ± 1.08 in the abaloparatide group, whereas it was 3.52 ± 1.25 and 3.27 ± 1.18 in the control group. The ICC for left-sided measurements was 0.861 (95% CI 0.712 – 0.939) and 0.831 (0.651–0.926) for the right-sided measurements respectively (Figure 3).
Figure 3:
Measured Bone Volume Formed from Quantitative Computed Tomography Scans. A. Bone Volume Formed of Fusion Mass. B. Radiographic Scores. C. Representative image of a fused abaloparatide treated animal. D. Representative image of a fused control treated animal. *** p<0.001.
HR-pQCT analysis revealed a greater volume of bone formed for abaloparatide treated animals with a BV of 1209 ± 543mm3 compared to 551 ± 152mm3 (p<0.001) (Figure 3). Representative images of fused samples are shown in Figure 3.
Compared to the control group, the abaloparatide group had significantly greater trabecular bone volume fraction (Tb.BV/TV, +17%) and trabecular thickness (Tb.Th, +17%) and lower specific bone surface (BS/BV, −20%) and connectivity density (Conn.D, −22%). Abaloparatide treatment did not impact trabecular number (Tb.N) or separation (Tb.Sp) (Figure 4).
Figure 4:
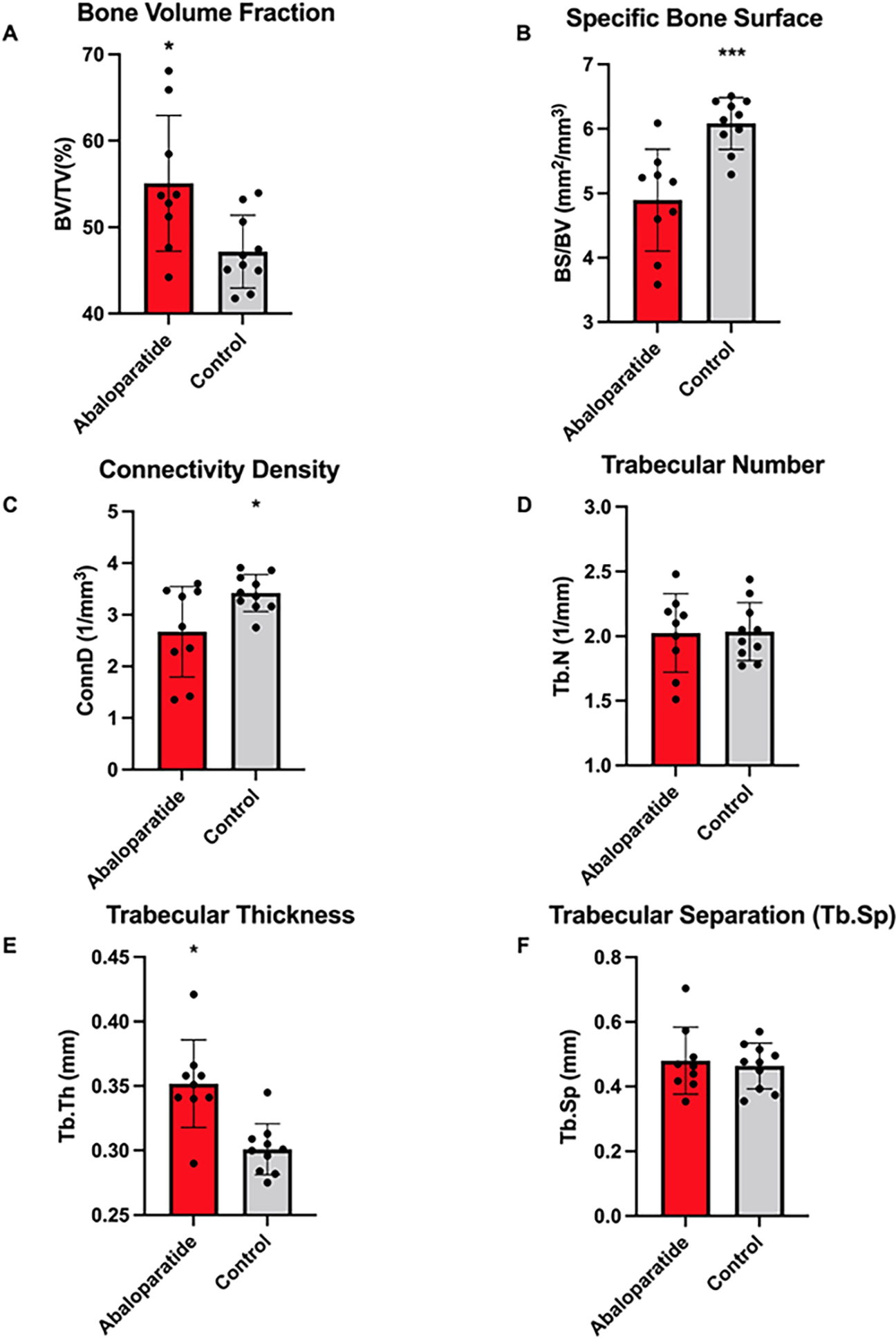
Plots of trabecular bone microarchitecture results. Plots are mean ± SD with the individual data points overlaid. A. Bone Volume Fraction. B. Specific Bone Surface. C. Connectivity Density. D. Trabecular Number. E. Trabecular Thickness. F. Trabecular Separation. *p<0.05, ***p<0.001.
Biomechanical Analysis
There were no differences in bending stiffness in either flexion, extension, right lateral bending, or left lateral bending between groups (p>0.05). The control group reached a bending stiffness of 4.1 ± 6.4 N/m2 in flexion, 7.25±2.5 N/m2 in extension, 7.6±1.5 N/m2 in left bending, and 7.2±1.5 N/m2 in right bending. The abaloparatide group reached a bending stiffness of 5.2 ± 1.9 N/m2 in flexion, 6.2 ± 1.2 N/m2 in extension, 7.9 ± 1.8 N/m2 in left lateral bending, and 8.6 ± 1.7 N/m2 in right lateral bending (Figure 5).
Figure 5:
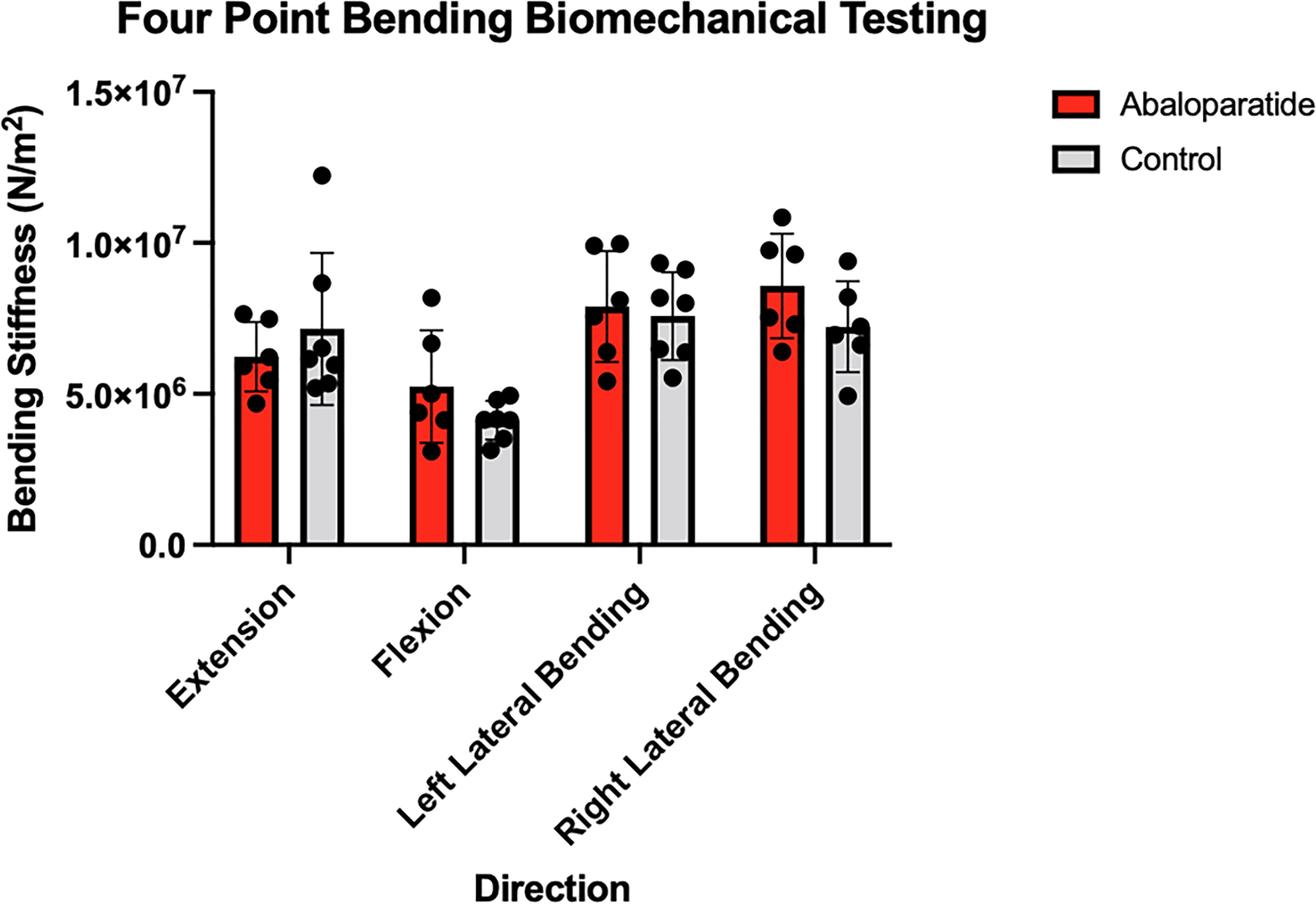
Bending Stiffness in a Four Point Bending Model. Plots are mean ± SD with the individual data points overlaid.
Discussion
In this rabbit posterolateral fusion model comparing abaloparatide treatment to saline control, we found a significantly increased rate of spine fusion assessed by manual palpation. Additionally, there were marked increases in the radiographic assessment of fusion as well as greater bone volume formed on HR-pQCT analysis. Further CT analysis revealed significantly greater trabecular bone volume fraction, trabecular thickness, and lower specific bone surface and connectivity density in the adjacent cranial vertebra for abaloparatide treated rabbits. Abaloparatide treatment did not impact trabecular number or separation, and no differences were found in the four-point bending model. These results show that abaloparatide treatment is efficacious as a medical adjunct in a rabbit spine posterolateral spine fusion model.
While many prior studies have utilized various formulations of intermittent PTH 1–34 as a medical adjunct to posterolateral spine fusion, this is the first study to utilize abaloparatide in rabbits. Recently, Arlt et al.17 reported on the use of abaloparatide on a rat posterolateral spine fusion model. Based on manual assessment, the authors reported a 50% fusion rate among abaloparatide animals compared to 25% of control animals at 28 days.17 We used a surgical protocol similar to O’Loughlin et al.9, who reported a manual assessment rate of 81% in PTH(1–34) treated animals compared to 30% of control animals. Similarly, we used an iliac crest graft volume of 1 cm3 to show a potential treatment effect of abaloparatide. We had a 100% fusion rate with all raters showing concordance of rating fusion in 9/10 animals. We do report a 45% fusion rate amongst control animals, which is slightly higher compared to O’Loughlin et al9 but similar to Lina et al.7 and Lehman et al.8 in their rabbit models, which validates the current model.
In addition to the manual assessment of fusion, animals treated with abaloparatide had higher mean radiographic fusion scores, and HR-pQCT analysis assessment revealed a more significant amount of bone volume formed. The radiographic scores are similar to that of O’Loughlin et al.9 The use of radiographs in the evaluation of fusion are essential, as clinically, surgeons often utilize radiographs to assess fusion in the early postoperative period.
Cranial adjacent vertebrae showed increases in trabecular bone volume fraction, trabecular thickness, and lower specific bone surface and connectivity density demonstrating the ability of abaloparatide to positively affect adjacent vertebrae bone mass. Successful spine fusion creates a rigid construct, which may increase forces distributed across the adjacent vertebrae. Patients with pre-existing osteoporosis are at increased risk of adjacent vertebral fracture following spine fusion.23–26 As abaloparatide is a systemic drug, all skeletal elements are positively affected. These additional measurements show that abaloparatide also improves the vertebral trabecular bone architecture adjacent to the fusion, therefore providing a potential additional benefit.
This study has several limitations. Scans provided to BIDMC were not calibrated for mineral density, so it was not possible to measure the bone mineral density of the trabecular bone. Additionally, we did not report an increase in bending stiffness following biomechanical testing. This result is similar to other animals model that utilized biomechanical testing following administration of PTH(1–34).7,8,12 In a posterolateral rabbit fusion model studying the effects of PTH(1–34), Lina et al.7 reported both increased fusion bone volume as assessed by CT compared to control but no differences in biomechanical stiffness. Additionally, in their study, Lehman et al.8 reported increased in histologic fusion scores following administration with teriparatide but no differences in biomechanical assessment. Biomechanical testing is often not universally reported in small animal studies given the variable results and the manual assessment of fusion is the standard to assess rabbit posterolateral fusion.9,21 The current study was powered with a primary outcome of a manual assessment of fusion and may have been underpowered to detect a difference in bending stiffness following biomechanical testing. Additionally, we used a 6 week harvest time point, and more time may be required to demonstrate a biomechanical difference, as Chandler et al.27 reported reported restored femoral diaphysis bending strength following abaloparatide administration in an osteoporosis induced rabbit model. We also did not report histological or serologic endpoints. Arlt et al.17 reported increases in serum osteocalcin and no differences in TRACP-5B for abaloparatide treated rats compared to controls suggestive of systemic osteoblastic stimulation without bone resorption.
PTH(1–34) has been studied as a systemic adjunct for spine fusion in osteoporotic patients with reportedly increased fusion rates and bone mineral density.13,14,28,29 While PTH(1–34) is able to increase bone density, it also may increase bone resorption16,30,31 Abaloparatide has been shown previously to increase bone mineral density to include cortical thickness and trabecular volume to nearly reverse an osteoporosis phenotype in rats without increasing bone resorption offering a potential advantage of abaloparatide compared to PTH(1–34).16 Additionally, it has been shown to reduce the risk of vertebral and non-vertebral osteoporotic fractures in humans.32
In conclusion, abaloparatide treatment significantly enhanced spine fusion outcomes as assessed by manual palpation as well as adjacent segment trabecular bone architecture. Future study is warranted to evaluate spine fusion outcomes in the human population.
Key Points.
Rabbits that received abaloparatide had a significantly increased fusion rate assessed by manual palpation compared to controls.
Abaloparatide treatment markedly increased fusion mass as measured by x-ray and computed tomography.
Systemic abaloparatide treatment improved trabecular bone architecture.
Acknowledgements:
Abaloparatide and funding for this study were provided by Radius Health (Boston, Massachusetts). Kyle W. Morse, MD was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number T32-AR078751. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Rajaee SS, Bae HW, Kanim LEA, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine. 2012;37(1):67–76. doi: 10.1097/BRS.0b013e31820cccfb [DOI] [PubMed] [Google Scholar]
- 2.Sheikh SR, Thompson NR, Benzel E, et al. Can We Justify It? Trends in the Utilization of Spinal Fusions and Associated Reimbursement. Neurosurgery. 2020;86(2):E193–E202. doi: 10.1093/neuros/nyz400 [DOI] [PubMed] [Google Scholar]
- 3.Buser Z, Ortega B, D’Oro A, et al. Spine Degenerative Conditions and Their Treatments: National Trends in the United States of America. Global Spine J. 2018;8(1):57–67. doi: 10.1177/2192568217696688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajaee SS, Kanim LEA, Bae HW. National trends in revision spinal fusion in the USA: patient characteristics and complications. Bone Joint J. 2014;96-B(6):807–816. doi: 10.1302/0301-620X.96B6.31149 [DOI] [PubMed] [Google Scholar]
- 5.Singh K, Nandyala SV, Marquez-Lara A, Fineberg SJ. Epidemiological trends in the utilization of bone morphogenetic protein in spinal fusions from 2002 to 2011. Spine. 2014;39(6):491–496. doi: 10.1097/BRS.0000000000000167 [DOI] [PubMed] [Google Scholar]
- 6.Ming N, Cheng JTY, Rui YF, et al. Dose-dependent enhancement of spinal fusion in rats with teriparatide (PTH[1–34]). Spine (Phila Pa 1976). 2012;37(15):1275–1282. doi: 10.1097/BRS.0b013e31824ac089 [DOI] [PubMed] [Google Scholar]
- 7.Lina IA, Puvanesarajah V, Liauw JA, et al. Quantitative study of parathyroid hormone (1–34) and bone morphogenetic protein-2 on spinal fusion outcomes in a rabbit model of lumbar dorsolateral intertransverse process arthrodesis. Spine (Phila Pa 1976). 2014;39(5):347–355. doi: 10.1097/BRS.0000000000000169 [DOI] [PubMed] [Google Scholar]
- 8.Lehman RA, Dmitriev AE, Cardoso MJ, et al. Effect of teriparatide [rhPTH(1,34)] and calcitonin on intertransverse process fusion in a rabbit model. Spine (Phila Pa 1976). 2010;35(2):146–152. doi: 10.1097/BRS.0b013e3181b71a96 [DOI] [PubMed] [Google Scholar]
- 9.O’Loughlin PF, Cunningham ME, Bukata SV, et al. Parathyroid hormone (1–34) augments spinal fusion, fusion mass volume, and fusion mass quality in a rabbit spinal fusion model. Spine (Phila Pa 1976). 2009;34(2):121–130. doi: 10.1097/BRS.0b013e318191e687 [DOI] [PubMed] [Google Scholar]
- 10.Abe Y, Takahata M, Ito M, Irie K, Abumi K, Minami A. Enhancement of graft bone healing by intermittent administration of human parathyroid hormone (1–34) in a rat spinal arthrodesis model. Bone. 2007;41(5):775–785. doi: 10.1016/j.bone.2007.06.025 [DOI] [PubMed] [Google Scholar]
- 11.Lawrence JP, Ennis F, White AP, et al. Effect of daily parathyroid hormone (1–34) on lumbar fusion in a rat model. Spine J. 2006;6(4):385–390. doi: 10.1016/j.spinee.2005.10.010 [DOI] [PubMed] [Google Scholar]
- 12.Holmes CA, Ishida W, Elder BD, et al. The Effects of High-Dose Parathyroid Hormone Treatment on Fusion Outcomes in a Rabbit Model of Posterolateral Lumbar Spinal Fusion Alone and in Combination with Bone Morphogenetic Protein 2 Treatment. World Neurosurg. 2018;115:e366–e374. doi: 10.1016/j.wneu.2018.04.058 [DOI] [PubMed] [Google Scholar]
- 13.Ebata S, Takahashi J, Hasegawa T, et al. Role of Weekly Teriparatide Administration in Osseous Union Enhancement within Six Months After Posterior or Transforaminal Lumbar Interbody Fusion for Osteoporosis-Associated Lumbar Degenerative Disorders: A Multicenter, Prospective Randomized Study. J Bone Joint Surg Am. 2017;99(5):365–372. doi: 10.2106/JBJS.16.00230 [DOI] [PubMed] [Google Scholar]
- 14.Ohtori S, Inoue G, Orita S, et al. Teriparatide accelerates lumbar posterolateral fusion in women with postmenopausal osteoporosis: prospective study. Spine (Phila Pa 1976). 2012;37(23):E1464–1468. doi: 10.1097/BRS.0b013e31826ca2a8 [DOI] [PubMed] [Google Scholar]
- 15.Varela A, Chouinard L, Lesage E, et al. One year of abaloparatide, a selective peptide activator of the PTH1 receptor, increased bone mass and strength in ovariectomized rats. Bone. 2017;95:143–150. doi: 10.1016/j.bone.2016.11.027 [DOI] [PubMed] [Google Scholar]
- 16.Chandler H, Lanske B, Varela A, et al. Abaloparatide, a novel osteoanabolic PTHrP analog, increases cortical and trabecular bone mass and architecture in orchiectomized rats by increasing bone formation without increasing bone resorption. Bone. 2019;120:148–155. doi: 10.1016/j.bone.2018.10.012 [DOI] [PubMed] [Google Scholar]
- 17.Arlt H, Besschetnova T, Ominsky MS, Fredericks DC, Lanske B. Effects of systemically administered abaloparatide, an osteoanabolic PTHrP analog, as an adjuvant therapy for spinal fusion in rats. JOR Spine. 2021;4(1):e1132. doi: 10.1002/jsp2.1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine. 1995;20(4):412–420. doi: 10.1097/00007632-199502001-00003 [DOI] [PubMed] [Google Scholar]
- 19.Curylo LJ, Johnstone B, Petersilge CA, Janicki JA, Yoo JU. Augmentation of spinal arthrodesis with autologous bone marrow in a rabbit posterolateral spine fusion model. Spine (Phila Pa 1976). 1999;24(5):434–438; discussion 438–439. doi: 10.1097/00007632-199903010-00004 [DOI] [PubMed] [Google Scholar]
- 20.Boden SD, Martin GJ, Morone M, Ugbo JL, Titus L, Hutton WC. The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar spine fusion. Spine (Phila Pa 1976). 1999;24(4):320–327. doi: 10.1097/00007632-199902150-00003 [DOI] [PubMed] [Google Scholar]
- 21.Yee AJM, Bae HW, Friess D, Robbin M, Johnstone B, Yoo JU. Accuracy and interobserver agreement for determinations of rabbit posterolateral spinal fusion. Spine (Phila Pa 1976). 2004;29(12):1308–1313. doi: 10.1097/01.brs.0000127184.43765.61 [DOI] [PubMed] [Google Scholar]
- 22.Cottrell JM, van der Meulen MCH, Lane JM, Myers ER. Assessing the stiffness of spinal fusion in animal models. HSS J. 2006;2(1):12–18. doi: 10.1007/s11420-005-5123-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St Jeor JD, Jackson TJ, Xiong AE, et al. Average Lumbar Hounsfield Units Predicts Osteoporosis-Related Complications Following Lumbar Spine Fusion. Global Spine J Published online November 23, 2020:2192568220975365. doi: 10.1177/2192568220975365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjerke BT, Zarrabian M, Aleem IS, et al. Incidence of Osteoporosis-Related Complications Following Posterior Lumbar Fusion. Global Spine J. 2018;8(6):563–569. doi: 10.1177/2192568217743727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meredith DS, Schreiber JJ, Taher F, Cammisa FP, Girardi FP. Lower preoperative Hounsfield unit measurements are associated with adjacent segment fracture after spinal fusion. Spine (Phila Pa 1976). 2013;38(5):415–418. doi: 10.1097/BRS.0b013e31826ff084 [DOI] [PubMed] [Google Scholar]
- 26.Li YC, Yang SC, Chen HS, Kao YH, Tu YK. Impact of lumbar instrumented circumferential fusion on the development of adjacent vertebral compression fracture. Bone Joint J. 2015;97-B(10):1411–1416. doi: 10.1302/0301-620X.97B10.34927 [DOI] [PubMed] [Google Scholar]
- 27.Chandler H, Brooks DJ, Hattersley G, Bouxsein ML, Lanske B. Abaloparatide increases bone mineral density and bone strength in ovariectomized rabbits with glucocorticoid-induced osteopenia. Osteoporos Int. 2019;30(8):1607–1616. doi: 10.1007/s00198-019-04999-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oba H, Takahashi J, Yokomichi H, et al. Weekly Teriparatide Versus Bisphosphonate for Bone Union During 6 Months After Multi-Level Lumbar Interbody Fusion for Osteoporotic Patients: A Multicenter, Prospective, Randomized Study. Spine (Phila Pa 1976). 2020;45(13):863–871. doi: 10.1097/BRS.0000000000003426 [DOI] [PubMed] [Google Scholar]
- 29.Cho PG, Ji GY, Shin DA, Ha Y, Yoon DH, Kim KN. An effect comparison of teriparatide and bisphosphonate on posterior lumbar interbody fusion in patients with osteoporosis: a prospective cohort study and preliminary data. Eur Spine J. 2017;26(3):691–697. doi: 10.1007/s00586-015-4342-y [DOI] [PubMed] [Google Scholar]
- 30.Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18(1):9–17. doi: 10.1359/jbmr.2003.18.1.9 [DOI] [PubMed] [Google Scholar]
- 31.Miyauchi A, Matsumoto T, Sugimoto T, Tsujimoto M, Warner MR, Nakamura T. Effects of teriparatide on bone mineral density and bone turnover markers in Japanese subjects with osteoporosis at high risk of fracture in a 24-month clinical study: 12-month, randomized, placebo-controlled, double-blind and 12-month open-label phases. Bone. 2010;47(3):493–502. doi: 10.1016/j.bone.2010.05.022 [DOI] [PubMed] [Google Scholar]
- 32.Miller PD, Hattersley G, Riis BJ, et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA. 2016;316(7):722–733. doi: 10.1001/jama.2016.11136 [DOI] [PubMed] [Google Scholar]