1. Introduction
The Complications After Major and Minor Urological Surgery (CAMUS) Collaboration is a worldwide collaborative initiative that was created to address the shortcomings of current standards in complication reporting and grading in urology. Accurate and standardised reporting and grading in hospital systems have immense clinical, academic, and financial potential. The CAMUS reporting and classification system was proposed to fill this void for recording and holistic assessment of a patient’s entire operative and postoperative morbidity within a single system [1]. Concurrent Delphi studies assessing the opinions of urologist, critical care specialist, and nursing groups [2] are under way and will provide valuable insight from chief stakeholders. We propose a classification system with a main CAMUS grade, four supplementary grades, and a CAMUS comprehensive complication index (CCI) based on worldwide expert opinion [3]. In combination, these grades were developed to ensure essential coverage of the full spectrum of potential complications in the short, medium, and long term (beyond 90 d).
For the all-inclusive CAMUS system to be considered comprehensive and reach its full potential, broad uptake by the wider urological community is essential. Only then will the classification system provide holistic benefit to all stakeholders (eg, surgeons, patients, next of kin, researchers, and insurance companies) via long-term acquisition of data to improve comparisons and performance in delivering surgical care [4].
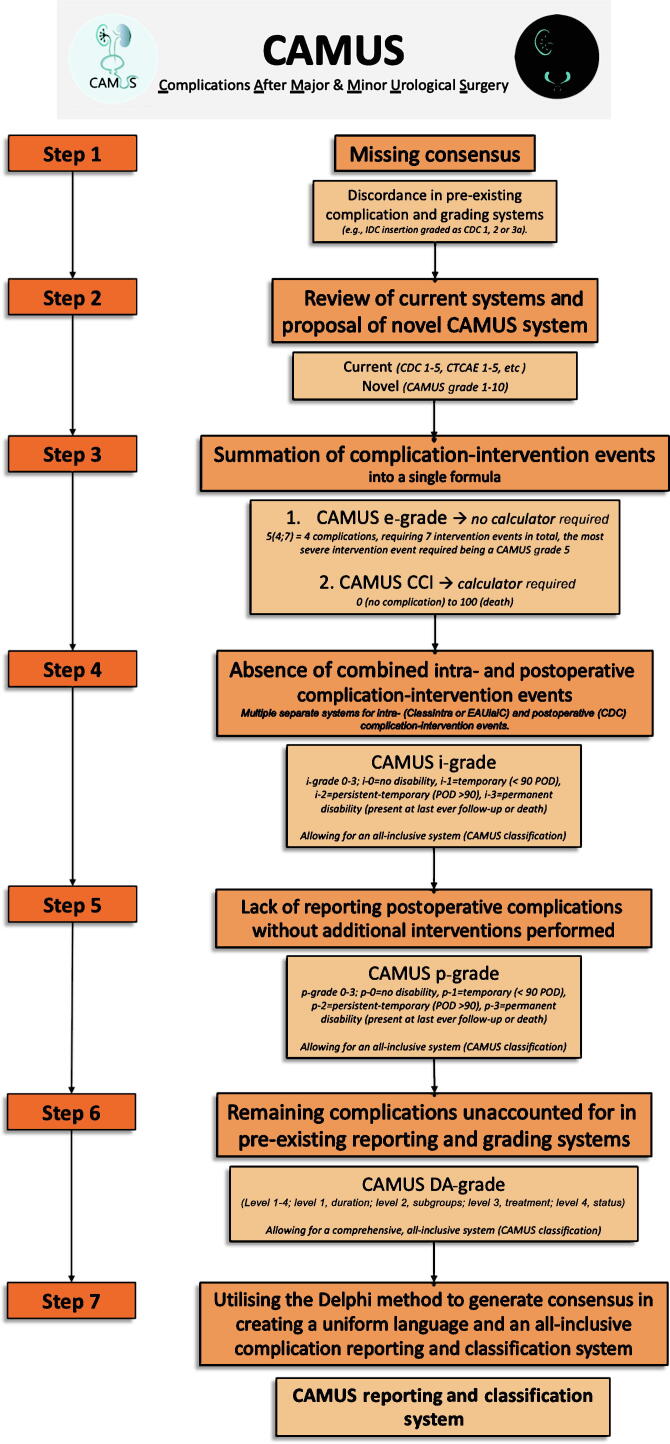
An appreciation of the basis for its rationale and the ensuing step-by-step evolution is first necessary, as well as a clear understanding of the various CAMUS classification components, so these are presented in Figure 1.
Fig. 1.
Step-by-step development of the CAMUS reporting and classification system from rationale to end product. Starting from the lack of consensus on how to report and grade various complication-intervention events and the absence of a single system that incorporates all intraoperative and postoperative events, the comprehensive, all-inclusive CAMUS reporting and complication system was established by applying a method of elimination. IDC = indwelling catheter; CDC = Clavien-Dindo classification; CTCAE = Common Terminology Criteria for Adverse Events; CCI = comprehensive complication index; POD = postoperative day; DA = disability adjunct.
2. Step 1: lack of consensus
A large, prospectively maintained, single-centre clinical database with comprehensive postoperative follow-up data was used to initially identify and define pitfalls of pre-existing standards for complication reporting and grading. For example, there is inconsistent classification of insertion of an indwelling urinary catheter, with Clavien-Dindo classification (CDC) [5] varying from grade 1 to 2 to 3a; although this is frequently a bedside procedure, it is invasive and local anaesthetic gel is required. Other examples include endoscopic procedures (eg, suprapubic catheter insertion, flexible cystoscopy, and flexible or rigid conduitoscopy) that can be performed using local anaesthetic gel or spinal or general anaesthesia (inconsistently graded as CDC grade 2, 3a or 3b), and complex wound management (eg, exchange of vacuum-assisted closure dressings) that can be performed under oral sedation, local anaesthesia, spinal anaesthesia, or general anaesthesia, or without anaesthesia at all (variably graded as CDC grade 1, 2, 3a or 3b).
This lack of consistency in grading of adverse events inherently discredited any attempt to confidently use such data for internal audit or quality assessment purposes. Thus, we recognised a need for uniformity in language and terminology to drive progress in the field [4].
3. Step 2: review of current classification systems and proposal of a novel reporting and grading tool for complications
Reassessment of the CDC, the core classification system used in urology and other surgical specialities, was necessary to understand why current methods for grading never prompted local or international standardisation of surgical care. Multiple pitfalls in the CDC were identified (Table 1) and solutions involving adaptation of the system were proposed. However, given our primary goal of creating uniformity and standardisation, attempts to both alter and augment a pre-existing system resulted in inconsistencies and contradictions.
Table 1.
Major pitfalls of the Clavien-Dindo Classification
| Pitfalls | |
|---|---|
| 1 | Only reports the most severe intervention for any given complication that occurs and thus fails to recognise cumulative patient morbidity. |
| 2 | Purely an intervention-based system (ie, does not report complications that do not require interventions). |
| 3 | Only recognises postoperative complications and fails to report intraoperative events. |
| 4 | Severity is predominately defined by grade of anaesthesia. |
| 5 | Does not differentiate between early and late postoperative complications (only reports complications that occur within 90 d postoperatively). |
| 6 | Inter-rater variability is reported to be significant. |
| 7 | Not validated in paediatric populations. |
| 8 | Fails to assess both patient-reported outcome measures and patient-reported experience measures, and thus does not consider the impact of complications and overall satisfaction with care from the patient perspective. |
To overcome these issues, a completely novel CAMUS system was proposed. This intervention-based system encompasses an easy and simplistic CAMUS grade (on a scale from 1 to 10) at its core that provides greater bandwidth for differentiation between discrete levels of severity. The CAMUS grade provided a basis for the creation of our user-friendly and reproducible grading system and complements the supplementary grades proposed.
4. Step 3: simplified integration of all complication-intervention events within a single grade
Integration all patient-specific complications into a single value, as previously proposed with the CCI and the Bern CCI [6], is a valuable outcome. This allows efficient and comparable quantification of the overall patient burden. A CAMUS CCI will be developed using consensus-derived values on a scale from 0 to 100 for all individual complication-intervention (C/I) events provided in the Delphi questionnaire, in combination with nurse opinion from the Delphi process and patient opinion. This index will summarise and track all C/I events for a single patient after urological surgery in the short, medium, and long term.
Unfortunately, calculation of the CCI without the aid of a computer appears to be impossible. As a result, we proposed the creation of a simplified CAMUS extended grade (e-grade). The e-grade includes the most severe grade in combination with the total number of C/I events occurring postoperatively. The e-grade will act as a simplified summative representation of patient morbidity and provide rapid and important additional information without the use of an online tool.
5. Step 4: absence of reporting systems that include both intraoperative and postoperative complications
It has been noted that intraoperative adverse events should not be graded according to the CDC; however, the use of different reporting (European Association of Urology [EAU] Guideline Panel Criteria [7] for postoperative events and ICARUS [8] for intraoperative events) and grading (CDC [5] for postoperative events and EAUiaiC [9] or ClassIntra [10] for intraoperative events) systems to gather complications for the same patients is suboptimal. Thus, the idea to create a simplified intraoperative grading tool (CAMUS intraoperative grade [i-grade]) within the CAMUS system evolved.
Furthermore, the definition of individual intraoperative complications can be ambiguous and there is significant risk of subjectivity bias. The CAMUS i-grade defines intraoperative complications on the basis of the presence and duration of disability (no disability vs temporary, persistent-temporary, or permanent disability). This i-grade would ensure comprehensive intraoperative grading of complications not captured by postoperative grades. Although grade i-0 may be dependent on surgeon honesty, grades i1–i3 are objective and may be impartially assigned by an independent proctor.
6. Step 5: lack of reporting of postoperative complications for which no additional interventions are performed
It was noted that postoperative complications causing morbidity but for which active treatment is not required, or not wanted, have never been included in any reporting system. As complications without additional treatment should not be combined with complications requiring further interventions (either intraoperatively or postoperatively), a new four-level subgrade, the CAMUS postoperative grade [p-grade], was proposed to prevent underestimation of morbidity. Although untreated, such complications may still prove bothersome.
7. Step 6: remaining complications unaccounted for by pre-existing reporting and grading systems
Even after integration of the aforementioned variety of complications in new grading systems, further deviations from a normal postoperative course (ie, those requiring frequent minor interventions) that have not been reported in a standardised manner remain neglected. Failure to report these C/I events would underestimate overall patient morbidity and result in an incomplete complication reporting and grading system. To account for this omission, the four-tiered CAMUS disability adjunct (DA-grade) was proposed to reflect disability duration, subgroup classification, treatment, and status. This grade allows inclusion of the entire spectrum of minor interventions used to treat various complications.
8. Step 7: using the Delphi methodology to achieve consensus in creating a uniform language and an all-inclusive complication system
There is considerable discordance in the reporting and grading of adverse events in urology. To achieve consensus and acceptance within the broader urological community, a worldwide Delphi study was launched. The primary objective of the study was to achieve consensus on complication reporting and create a comprehensive and reproducible uniform language. Importantly, consensus on how to report a C/I event, which events should be reported, and which grade of complication is appropriate was needed.
To encourage standardisation, all complications and C/I events should be reported prospectively within a single comprehensive system. The CAMUS reporting and grading system was proposed to bridge the recognised void in academic and clinical urology. The Delphi study provided an ideal platform for the introduction of the entire CAMUS classification. This system has potentially significant economic implications and may lead to improvements in surgical care and patient counselling.
9. Delphi challenges and potential future implications
Use of the Delphi methodology to develop the core CAMUS classification proved both beneficial and challenging. The main area of contention was in developing a single system that was all-inclusive yet still simple and user-friendly. Throughout the development of the Delphi pilot survey and review of the feedback, various new complication and C/I events were identified and several new CAMUS subgrades (eg, p-grade for complications without subsequent interventions) had to be created for integration of all the C/I events identified and to reflect the patient burden and the quality of surgical care. Other challenges included participant follow-up, given the significant time commitment required to complete the survey and the huge number of participants (>1100), and various logistics issues associated with survey distribution and the use of an online password-protected system.
A comprehensive system such as the CAMUS system has potentially significant implications in both surgery and research; however it must be reproducible, reliable, and implemented worldwide (Table 2). Global standardisation, validation, and acceptance of complication reporting and grading will not only be of enormous value to the field of medical research, allowing great opportunities to conduct prospective randomised and nonrandomised trials, but may also be calibrated to create reliable international guidelines and recommendations to benefit all stakeholders (surgeons, units, hospitals, patients, family members/next of kin, researchers, nurses, health insurance companies, politicians, urological organisations). This may ultimately lead to significant improvements in surgical quality and unit efficiency, and could provide valuable insight into intraoperative and postoperative morbidity to allow improvements in patient counselling and satisfaction.
Table 2.
Potential implications of the CAMUS classification in research and clinical practice
| Implications | |
|---|---|
| 1 | Stimulate competition between urologists and centres internationally. |
| 2 | Improvements in surgical quality and unit efficiency. |
| 3 | Provide a better understanding of intraoperative and post-operative morbidity. |
| 4 | Offer transparency for patient counselling regarding potential surgical morbidity. |
| 5 | Improve the accuracy and quality of patient consent. |
| 6 | Creation of an online grading and reporting tool and registry for surgeons to record and store all complications on a single worldwide anonymous database. |
| 7 | Potential for the worldwide anonymous database to be deanonymised, should government bodies desire, to audit complications for quality control or publish complication registry results to allow patients to compare centres and choose their preferred surgeon or facility. |
| 8 | Create an opportunistic window for a wide variety of clinical research, including prospective randomised and nonrandomised trials. |
Conflicts of interest: The authors have nothing to disclose.
References
- 1.Soliman C, Sathianathen NJ, Thomas BC, et al. A systematic review of intra- and postoperative complication reporting and grading in urological surgery: understanding the pitfalls and a path forward. Eur Urol Oncol. In press. 10.1016/j.euo.2023.01.002. [DOI] [PubMed]
- 2.Soliman C., Thomas B.C., Santaguida P., et al. Active involvement of nursing staff in reporting and grading complication-intervention events—protocol and results of the CAMUS pilot nurse Delphi study. BJUI Compass. 2022;3:466–483. doi: 10.1002/bco2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soliman C., Mulholland C.J., Santaguida P., et al. Protocol for CAMUS Delphi study: a consensus on comprehensive reporting and grading of complications after urological surgery. Eur Urol Focus. 2022;8:1493–1511. doi: 10.1016/j.euf.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Soliman C., Sathianathen N.J., Giannarini G., et al. There is a need for a universal language in the reporting and grading of complication and intervention events to ensure comparability and improvement of surgical care. Eur Urol. 2022;81:440–445. doi: 10.1016/j.eururo.2021.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furrer M.A., Huesler J., Fellmann A., Burkhard F.C., Thalmann G.N., Wuethrich P.Y. The Comprehensive Complication Index CCI: a proposed modification to optimize short-term complication reporting after cystectomy and urinary diversion. Urol Oncol. 2019;37:291.e9–291.e18. doi: 10.1016/j.urolonc.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Mitropoulos D., Artibani W., Graefen M., Remzi M., Rouprêt M., Truss M. Reporting and grading of complications after urologic surgical procedures: an ad hoc EAU guidelines panel assessment and recommendations. Eur Urol. 2012;61:341–349. doi: 10.1016/j.eururo.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Cacciamani G.E., Sholklapper T., Dell’Oglio P., et al. The Intraoperative Complications Assessment and Reporting with Universal Standards (ICARUS) global surgical collaboration project: development of criteria for reporting adverse events during surgical procedures and evaluating their impact on the postoperative course. Eur Urol Focus. 2022;8:1847–1858. doi: 10.1016/j.euf.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Biyani C.S., Pecanka J., Rouprêt M., Jensen J.B., Mitropoulos D. Intraoperative adverse incident classification (EAUiaiC) by the European Association of Urology ad hoc Complications Guidelines Panel. Eur Urol. 2020;77:601–610. doi: 10.1016/j.eururo.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Dell-Kuster S., Gomes N.V., Gawria L., et al. Prospective validation of classification of intraoperative adverse events (ClassIntra): international, multicentre cohort study. BMJ. 2020;370 doi: 10.1136/bmj.m2917. [DOI] [PMC free article] [PubMed] [Google Scholar]