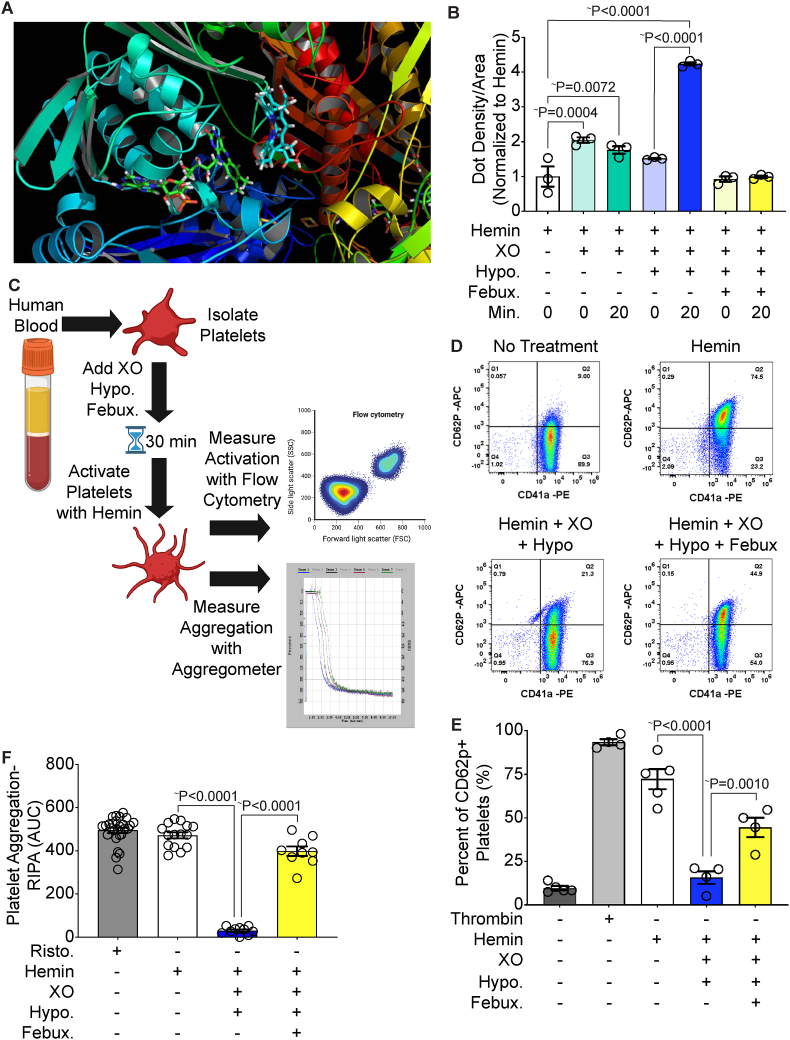
Fig. 5.
XO binds hemin and prevents platelet activation and aggregation. A) Molecular modeling of hemin and XO identified a predicted hemin binding site within the FAD domain of XO. B) Hemin-XO interaction was evaluated via a hemin binding dot blot. Reactions were incubated for 20 min and stopped with Laemelli buffer. Samples were added to a nitrocellulose dot blot and chemiluminescent detection was used to identify hemin binding. C) Schematic of platelet activation and aggregation experiments. Platelets were isolated from healthy human blood and incubated with XO, hypoxanthine and febuxostat for 30 min prior to stimulation with hemin. Platelet activation was measured using flow cytometry and platelet aggregation was measured using an aggregometer. This schematic was created using biorender.com. D-E) Platelets were stained with the platelet marker CD41a and the platelet activation marker CD62p to determine the percentage of activated platelets in the sample. Untreated platelets were used as a negative control and thrombin was used as a positive control. Quantification of the flow scatter plots in D are shown in E. F) Platelet aggregation was measured using an aggregometer and calculated as the AUC. Ristocetin was used as a positive control. ∼Values are mean ± SEM using a 1-way ANOVA with Sidak's multiple comparisons test. XO, xanthine oxidase; Hypo, hypoxanthine; Febux, febuxostat; Min, minute; RIPA, ristocetin-induced platelet activation; AUC, area under the curve; Risto, ristocetin.