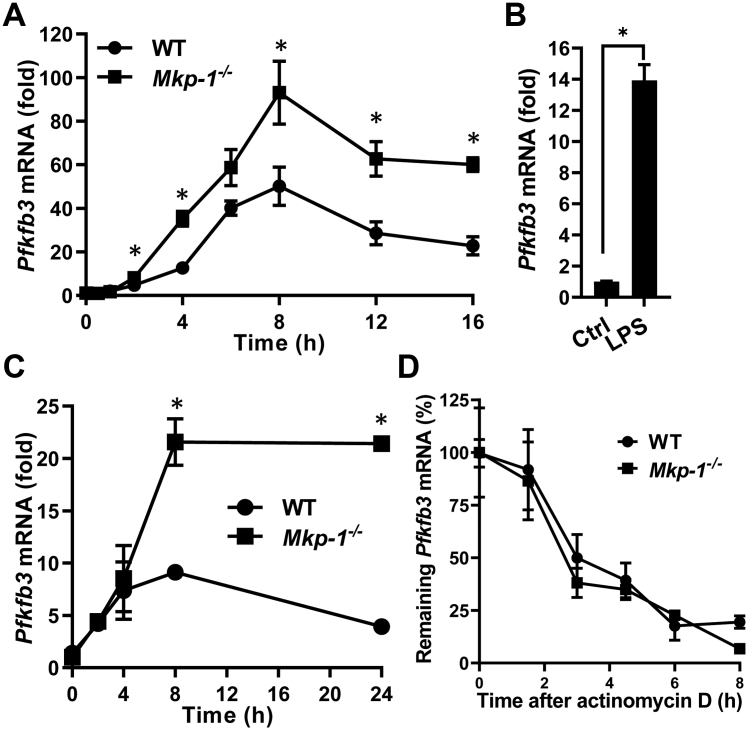
Figure 5.
Mkp-1 deficiency enhances Pfkfb3 mRNA expression in macrophages following inflammatory stimuli but has little effect on Pfkfb3 mRNA stability.A, kinetics of Pfkfb3 mRNA induction following Escherichia coli stimulation. WT and Mkp-1−/− BMDM were stimulated with heat-killed E. coli (MOI: 10:1) for different times. Pfkfb3 mRNA levels in the samples were quantitated by qRT-PCR. The results were normalized to 18S ribosomal RNA. The expression of mRNA is presented as fold change relative to control cells. ∗p < 0.05, compared to WT at the same time-point (t test, n = 6). B, induction of Pfkfb3 mRNA in WT BMDM by LPS. WT BMDM were treated with LPS for 6 h. ∗p < 0.05, compared to WT control (t test, n = 6). C, kinetics of Pfkfb3 mRNA induction in WT and Mkp-1−/− BMDM following LPS stimulation. WT and Mkp-1−/− BMDM were stimulated with 100 ng/ml LPS for different times. ∗p < 0.05, compared to WT at the same time-point (t test, n = 3). D, the decay of Pfkfb3 mRNA in WT and Mkp-1−/− BMDM after actinomycin D treatment. WT and Mkp-1−/− BMDM were stimulated with 100 ng/ml LPS for 8 h and then treated with 10 ng/ml actinomycin D. Cells were harvested at different times to quantitate Pfkfb3 mRNA levels using qRT-PCR. The values were normalized to 18S ribosomal RNA and expressed as percentage relative to levels prior to actinomycin D addition. The half-life of Pfkfb3 mRNA in WT and Mkp-1−/− BMDM was estimated to be 2.84 and 2.33 h, respectively. MKP, MAPK phosphatase; PFKFB, phosphofructo-2-kinase/fructose-2,6-biphosphatase; BMDM, bone marrow–derived macrophage; LPS, lipopolysaccharide; qRT-PCR, quantitative RT-PCR.