Abstract
Background
Deceased donor liver transplantation (DDLT) is increasing in India and now constitutes nearly one-third of all liver transplantation procedures performed in the country. There is currently no uniform national system of allocation of deceased donor livers.
Methods
A national task force consisting of 19 clinicians involved in liver transplantation from across the country was constituted under the aegis of the Liver Transplantation Society of India to develop a consensus document addressing the above issues using a modified Delphi process of consensus development.
Results
The National Liver Allocation Policy consensus document includes 46 statements covering all aspects of DDLT, including minimum listing criteria, listing for acute liver failure, DDLT wait-list management, system of prioritisation based on clinical urgency for adults and children, guidelines for allocation of paediatric organs and allocation priorities for liver grafts recovered from public sector hospitals.
Conclusion
This document is the first step in the setting up of a nationally consistent policy of deceased donor liver allocation.
Keywords: variant syndrome, acute liver failure, paediatric, public sector hospital, Delphi process
Abbreviations: ACLF, acute on chronic liver failure; ALF, acute liver failure; CLD, chronic liver disease; CSS, Clinical Severity Score; CSS-P, Clinical Severity Score for Paediatric Recipients; DD, deceased donation; DDLG, deceased donor liver grafts; DDLT, deceased donor liver transplantation; HCC, hepatocellular carcinoma; LDLT, living donor liver transplantation; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; NABL, National Accreditation Board for Testing & Calibration Laboratories; N-LAP, National Liver Allocation Policy; PELD, Paediatric Model for End-Stage Liver Disease; PuSH, Public Sector Hospital; WL, waiting list
Liver transplant activity in India has been increasing over the last decade. A total of 11,971 liver transplantations (LTs) were performed in India between 2013 and 2019, of which 3566 (29.8%) were deceased donor liver transplantations (DDLT).1 The Human Tissue and Organ Transplant Authority was legislated 28 years ago to establish the policies and standards for deceased donation (DD) in India, but donation rates have been low across the country. Southern and Western Indian states have shown a slow but consistent growth in organ donation, with rates increasing from 0.27 to 0.52 per million population from 2013 to 2019, although the DD numbers remain low compared to Western countries.1 Historically, allocation policy of deceased donor liver grafts (DDLGs) has been determined by individual states rather than via a national policy. Consequently, allocation policies have differed between states and sometimes even between individual hospitals within the same state. The Liver Transplantation Society of India recognises that although this arrangement may have been helpful in initially establishing DD and DDLT activity, there is now a clear need for a nationally consistent system of assessing liver transplant eligibility, waiting list (WL) prioritisation and DDLG allocation, as more states and hospitals come on board. The aim of this consensus document is to develop a system of liver allocation, which is fair to patients needing liver transplant and to families of donors who come forward to donate and considers specific issues relevant to health care provision in India.
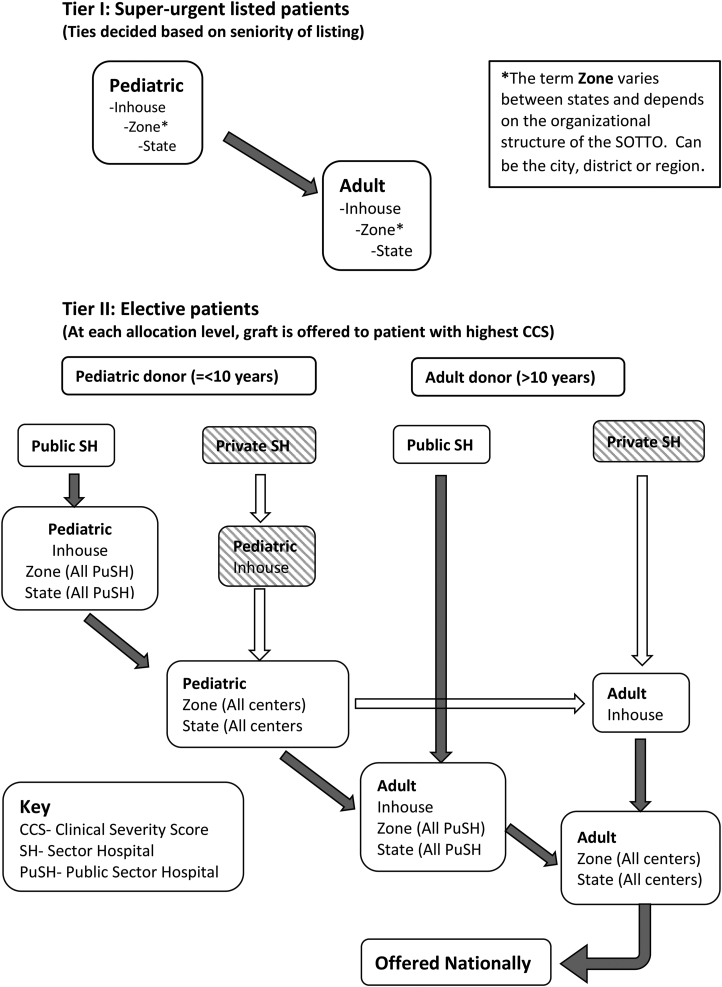
This consensus document was developed over a period of 9 months using a modified Delphi consultation process and involved 19 LT clinicians from across India.2 The consultation process was initiated by Dr. S. K. Mathur, Dr. Sudhindran S. and Dr. Mettu S. Reddy and included Dr. Arvinder Soin, Dr. Mohamed Rela, and Dr. Subhash Gupta as members of a steering committee formed for this purpose. The expert committee also included 11 additional Liver Transplantation Society of India members from different states and specialities. Members were chosen to ensure a mix of clinicians working in the public and private sector institutions. To get an international perspective, Dr. Darius Mirza from the United Kingdom and Dr. Vinay Kumaran from the United States were also invited to join the consultation process. The process included an extensive literature survey by the steering committee and by subgroups within the committee, four online questionnaire surveys and two virtual meetings amongst the participants. Recommendations were graded according to the GRADE system assessing both the strength of evidence (A-C) and the strength of consensus (strong-1 or weak-2).3 The present document is a summary of these discussions, and the proposed DDLG Allocation Pathway (Figure 1).
Figure 1.
Proposed deceased donor liver graft allocation cascade for N-LAP. N-LAP, National liver allocation policy.
General principles
DDLGs are a public resource generated through altruistic donations by the families of a deceased person, and naturally, their utilisation should follow the principles of equity, utility, justice and transparency. The ‘sickest first’ approach has been the primary basis for liver allocation in the United States since the passing of the Final Rule in 2000.4 The United Kingdom has now moved to a transplant benefit–based system, which includes clinical urgency as a variable in the allocation process.5 In India, the basis for prioritisation has varied between states and centres. Model for End-Stage Liver Disease (MELD) score has been used to prioritise patients in some centres but suffers from a lack of transparency. Waiting time has been a common basis for prioritisation in many centres, as it is a clear and simple metric. However, the urgency of LT is not always determined by the waiting time. This has led to a paradoxical situation where sick decompensated patients die before they accrue enough waiting time, while low MELD patients who could remain stable for a sufficient period on the WL undergo DDLT. The committee hence agreed that clinical urgency, defined as the risk of death or becoming too sick for transplant while on the waiting list, should be the primary criterion for allocating DDLG. Toward this goal, the use of a transparent disease severity–based system of prioritisation was considered the best way of using this scarce resource.
-
•
A nationally uniform system of wait-list prioritisation and allocation for DDLT is necessary. (A1)
-
•
DDLG allocation should be based on clinical urgency of patients on the waiting list. (A1)
‘Centre-based’ or ‘patient-based’ allocation
Despite their enormous potential, DD in Indian public sector hospitals (PuSHs) and private sector nontransplant hospitals is uncommon because of limitations of infrastructure, logistics and conflicting priorities. A large proportion of DD in India is facilitated in private health care institutions with active liver transplant programmes.6 A primary reason for this is the hospital management's commitment to support their LT programmes through in-house allocation of organs. The committee agreed that although this system of centre-based allocation is not ideal, immediately changing the status quo will lead to a significant reduction in DD as centres will lose the motivation to encourage donation within their hospitals. A purely patient-based allocation policy can only be successful when DD becomes a standard process in every hospital with an intensive care unit, including nontransplant centres.
The consensus hence was to have a hybrid solution, where the donor hospital has the first option to transplant a patient with the greatest clinical need within their waiting list. If the donor hospital does not have a suitable recipient for that organ, then the organ is allocated by open offer to a patient in the region with highest clinical need. The committee felt that this compromise solution will ensure that within each centre, sicker patients are offered DDLT preferentially, cold ischaemia time is kept short and hospitals remain committed to the organ donation effort.
-
•
In the current Indian scenario, where DD is largely driven by individual transplant centres, a hybrid system of liver graft allocation has the best chance of successful application. (B1)
-
•
A hybrid system requires that a DDLG is first allocated to in-house recipients of the donor hospital with greatest clinical urgency, followed by patients with the highest clinical urgency in the common waiting list of respectivezone/state/region. (B1)
DDLT indications and minimal listing criteria
Indications for LT are continuously evolving, and as the procedure becomes increasingly accepted and safe, expansion of transplant criteria will be inevitable.7 Although extended indications may be appropriate in the LDLT setting where a related donor makes a directed donation, DDLT listing should be considered only for patients who have a significant mortality risk without a transplant and proven satisfactory long-term benefit from LT. These include patients with acute liver failure (ALF), acute on chronic liver failure (ACLF), chronic liver disease (CLD) with decompensation or hepatocellular carcinoma (HCC) within well-defined criteria and selected other indications (Table 1). Similarly, in children, LT is indicated for ALF, cholestatic liver diseases such as biliary atresia, a variety of inherited metabolic diseases and liver tumours such as hepatoblastoma (Table 2). Internationally, most allocation systems have supported a two-tiered system with an urgent tier for ALF patients and a standard tier for CLD patients with an MELD score of ≥15 or other features of decompensation. This system is already being followed in many states in India. The urgent listing criteria are based on the UK's National Health Service Blood & Transplant (NHSBT) criteria for super-urgent LT with some modifications (Table 3).
Table 1.
Listing Criteria for Deceased Donor Liver Transplantation (Adults) – Elective.
Cirrhosis of any cause with
|
Autoimmune liver diseases
|
Noncirrhotic metabolic liver diseases correctable by liver transplantation
|
|
Retransplantation for chronic rejection/chronic graft dysfunction due to vascular complications or cholangiopathy |
Miscellaneous indications
|
|
HCC, hepatocellular carcinoma; MELD, Model for End-Stage Liver Disease; CTP, Child Turcotte Pugh; NET, Neuroendocrine Tumor.
Indications not listed above can still be considered for DDLT listing after being discussed by the local/regional liver scientific committee.
Table 2.
Listing Criteria for Deceased Donor Liver Transplantation (Paediatric) – Elective.
|
Chronic liver diseases with decompensation/hepatocellular carcinoma/refractory to medical therapy Biliary atresia Progressive familial intrahepatic cholestasis Autoimmune hepatitis Sclerosing cholangitis Caroli's disease Wilson's disease Cystic fibrosis Alagille's syndrome Glycogen storage disease Types 3 and 4 Tyrosinemia type 1 Budd-Chiari syndrome |
|
Liver tumours Unresectable hepatoblastoma after neoadjuvant chemotherapy Unresectable benign liver tumours with symptoms |
|
Noncirrhotic metabolic liver diseases correctable by liver transplantation Criggler Najar syndrome type 1 Primary hypercholesterolemia Urea cycle defects Organic acidemias Primary hyperoxaluria Porphyria |
| Retransplantation for chronic rejection/chronic graft failure/biliopathy |
DDLT, deceased donor liver transplantation.
Indications not listed above can still be considered for DDLT listing after being discussed by the local/regional liver scientific committee.
Table 3.
Proposed Criteria Listing for Super-Urgent Liver Transplantation (Modified From the Super-Urgent Listing Criteria Published by NHS Blood and Transplant).20
| • Liver failure in a living liver donor: Any patient who has been a living liver donor who develops severe liver failure within 4 weeks of the donor operation |
| • Severe early allograft dysfunction within 7 days of liver transplantation characterised by at least two of the following (AST >10,000, INR >3.0, arterial lactate >3 mmol/L, absence of bile production) |
| • Anhepatic patient after total hepatectomy, e.g., for trauma, etc. |
| • Paracetamol poisoning (Categories 1–4 in NHSBT super-urgent listing criteria) |
|
| • Favourable etiologies (eg, acute viral hepatitis) |
|
|
|
|
|
|
|
| • Unfavourable aetiologies (seronegative hepatitis, idiosyncratic drug reactions, drug-induced liver injury [DILI]) |
|
|
|
|
|
|
|
| • Ratol (yellow phosphorus) poisoning:30,31 Patients meeting any of the following three criteria |
|
|
|
| • Hepatic artery thrombosis (HAT): HAT within 21 days of liver transplantation |
| • Acute presentation of Wilson's disease, autoimmune hepatitis or Budd-Chiari syndrome with a combination of coagulopathy and encephalopathy |
| • Acute liver failure in children (under 2 years of age): INR >4 and/or grade 3–4 encephalopathy |
MELD, Model for End-Stage Liver Disease; AST, Aspartate aminotransferase; ICP, Intra-cranial pressure; INR, International normalised ratio; NHS, National health service; NHSBT, National health service blood & transplant; HE, Hepatic encephalopathy.
ACLF is becoming a frequent indication for LT in India.8 While clinically distinct from ALF, patients with ACLF can have a rapidly deteriorating clinical course, and LT should ideally be considered within 7–10 days for best outcomes.9 The committee discussed the possibility of having an additional tier of urgency between ALF and CLD to cater to ACLF patients. However, this was not considered appropriate due to the lack of a universally accepted definition of ACLF, the complexities of policing such an additional tier and the impact of using a large number of liver grafts for ACLF patients. The feasibility of adopting a ‘Share 35’-like policy of priority allocation to high MELD patients across the state was also discussed. Committee members agreed that such a policy may lead to one-way allocation of grafts to a few large volume centres with a large recipient base, irrespective of their own ability to generate in-house donations. Instead, state-wide priority allocation should be limited to patients with ALF needing emergency LT. The committee agreed that patients with ACLF or acute deterioration should receive temporary prioritisation points based on the number of organ failures, which is the predominant predictor of early mortality. Many such patients will continue to undergo LDLT as that is the best option for timely LT in India at present.
-
•
Adult patients wait listed for DDLT for decompensated cirrhosis should have a laboratory MELD score equal to or greater than 15 unless they fulfil other indications for LT. (A1)
-
•
Patients with HCC withinUniversity of California San Francisco (UCSF)liver transplant criteria and unsuitable for curative therapies such as resection should be considered for DDLT listing irrespective of MELD score. (A1)
-
•
Patients listed for ALF will continue to be listed according to the modified NHSBT criteria for super-urgent LT listing (A1)
-
•
Patients with ACLF who need early LT should be considered for standard DDLT listing with additional priority points based on the number of failing organs or for living donor LT. (B1)
Development of a clinical severity score for wait-list prioritisation and organ allocation
The pros and cons of using a single or a composite score for wait-list prioritisation were extensively debated. Although MELD score is being used for prioritisation in several countries,13, 14, 15 its limitation in predicting wait-list mortality and posttransplant survival are well recognised.16,17 The United Kingdom has now moved to a transplant benefit model of prioritisation, which includes 21 recipient criteria instead of the four laboratory parameters used for MELD calculation.5 Committee members also highlighted the limitations of using MELD alone, including accuracy of reported laboratory reports, especially in tier 2 and 3 towns, need for frequent recalculation, insensitivity of MELD scores to complications such as spontaneous bacterial peritonitis, encephalopathy and hepatopulmonary syndrome and the difficulty in prioritising LT indications not associated with an elevated MELD score. Although the Child-Pugh score was considered as an alternative, its lack of objectivity and sensitivity to changes in clinical status made it unsuitable as a listing and prioritisation tool. A composite score called the Clinical Severity Score for Adult Recipients and Clinical Severity Score for Paediatric Recipients (CSS-P), including MELD or Paediatric Model for End-Stage Liver Disease (PELD) score, cirrhosis complications, acute deterioration and waiting time, was hence considered (Table 4).
Table 4.
Proposed Clinical Severity Score-Adult (CSS-A). CCS-A score = MELD points + cirrhosis complications points + HCC points + acute deterioration points + waiting time points (maximum possible score is 40). MELD points = actual MELD score/2 (rounded off). Points capped at 20 (ie, MELD of 40).
| Cirrhosis complications points – Maximum of three complications can be considered | |
|---|---|
| Complications | Points added |
| Variceal bleed causing decompensation | 1 |
| Portopulmonary hypertension | 1 |
| Frequent LVP (at least once monthly) | 1 |
| Episode of SBP | 2 |
| Hepatic hydrothorax | 2 |
| Recurrent encephalopathy needing hospital admission | 2 |
| Severe HPS (PaO2 <60 mm Hg in room air) | 2 |
| Hepatocellular carcinoma | Points added |
|---|---|
| Multifocal within UCSF or a single lesion >3 cm (AFP <1000, no macrovascular invasion, no extrahepatic metastasis) | 2 |
| Acute deterioration points | |
|---|---|
| Acute deterioration with organ failure (From Moreau et al.32) | |
| Kidney: S. Creatinine >2.0 mg/dL or need for RRT | |
| Brain: West Haven Grade 3–4 | |
| Heart: need for Vasopressors | |
| Lungs – PaO2/FiO2 <200 or need for mechanical ventilation | |
| Single organ | 2 |
| Two organs | 4 |
| More than two organs | 6 |
| Exception indications (MELD points, if actual MELD score is less than 15) | |
|---|---|
| Indication | Points added |
| PSC with cholangitis | 8 |
| PBC with pruritus | 8 |
| Retransplant for chronic rejection | 8 |
| Multiorgan transplant | 8 |
| Symptomatic PCLD, non-HCC liver tumours, metabolic liver disease | Actual MELD |
| Waiting time points | |||||
|---|---|---|---|---|---|
| Waiting time (months) | 0–3 | 3–6 | 6–9 | 9–12 | >12 |
| Points | 0 | 1 | 2 | 4 | 6 |
AFP, Alfa-feto-protein; HCC, hepatocellular carcinoma; HPS, hepatopulmonary syndrome; MELD, Model for End-Stage Liver Disease; LVP, Large volume paracentesis; PBC, Primary biliary cholangitis; PCLD, Polycystic liver disease; PSC, Primary sclerosing cholangitis; RRT, Renal replacement therapy; SBP, Spontaneous bacterial peritonitis.
MELD or PELD score was given the highest weightage (50%) in the composite score in line with consensus within the committee members. Cirrhosis complications were ranked as high risk, low risk or no additional risk of mortality based on available evidence and consensus amongst committee members. Points were allocated to each complication for up to a maximum of three complications. Additional points for new-onset organ failure were also included for score calculation to prioritise patients with acute deterioration on waiting list or presenting initially with ACLF.
Diseases such as Primary Sclerosing Cholangitis (PSC) or Primary Biliary Cholangitis (PBC) are considered as variant syndromes for organ allocation in the United Kingdom. Symptoms and prognosis in these patients do not correlate with laboratory MELD score, and hence, alternative means of prioritisation within the combined LT waiting list are necessary. For this purpose, selected indications were allocated MELD exception points weighted on their expected risk of mortality based on evidence from the allocation systems in the United States and the United Kingdom and consensus within the expert committee. Such patients will continue to receive additional points for cirrhosis complications, HCC or acute deterioration as with other indications.
The committee recognised that prioritisation of liver allocation based purely on the sickest first policy will disincentivise patients with intermediate MELD scores. Additional points to patients based on their waiting time may alleviate this situation, especially for patients who have a higher waiting time mortality in the medium term, that is, around 6–12 months. The consensus was to allot points proportional to the waiting time after an initial 3 months. The waiting time points will be capped beyond 12 months. The Clinical Severity Score for Adult Recipients score will be capped at 40 points.
-
•
Prioritisation on waiting list should be made using a composite clinical severity score (CSS) with weightage given to MELD score, cirrhosis-related complications, HCC, new-onset number of organ failures and waiting time. (B1)
-
•
For indications where MELD score does not reflect true clinical urgency, exception points will be allocated for each such diagnosis. (B1)
-
•
Waiting time points will help ensure that patients with MELD scores in the intermediate range are not excessively disadvantaged by the ‘sickest first’ policy. (C2)
-
•
Waiting time points will accrue after an initial waiting time of 3 months and capped beyond 12 months. (C2)
LT for HCC
LT is an important treatment option for HCC. Recent advances in locoregional therapy means that a small single HCC does not increase wait-list mortality significantly to justify additional urgency points. However, single tumours larger than 3 cm or multicentric HCC within UCSF criteria need prioritisation to minimise the risk of progression to beyond the waiting list criteria. Although there is increasing evidence that LDLT for tumours beyond UCSF criteria is associated with good outcomes,18,19 the committee agreed that at the present time, DDLT should be offered only to patients with HCC within UCSF criteria without major vascular invasion, high AFP (>1000) or extrahepatic disease.
-
•
Patients with HCC within UCSF criteria will be given additional points if the tumours are multifocal or at least one lesion is equal to or larger than 3 cm in diameter. (B2)
Frequency of clinical severity score recalculation
As the clinical condition of wait-listed patients is dynamic, CSS should be revised at regular intervals to capture changes in the clinical condition of the patient. The frequency of revision will depend on the MELD score at listing or the most recent review, with higher scores indicating the need for more frequent revisions. The responsibility of revision lies with the transplant centre and should be performed at least 72 h before any organ allocation based on that score.
Patients who are listed with a high CSS based on high MELD score will need reconfirmation of the high MELD score at regular intervals, failing which the score may be downgraded to the next lower tier. Similarly, patients who receive acute decompensation points will need to be renewed every week by the transplant centre. Patients who are listed for a super-urgent transplant should have their status renewed every 24 h to make sure that they continue to satisfy the super-urgent listing criteria and are considered fit for LT by the treating team.
-
•
Patients on the waiting list should have their MELD score recalculated at regular intervals to adjust the CSS. The frequency of recalculation should be based on the most recent MELD score for that patient. The minimum frequency of testing is MELD >30 weekly, 20–30 monthly, <20 every 3 months. (B2)
-
•
Additional acute decompensation points given to patients listed on the elective DDLT waiting list should be renewed at least weekly, providing evidence of continued need for organ support and pertinent blood investigations performed within 24 h of application for renewal. (B1)
-
•
Patients wait listed for super-urgent DDLT should have their status renewed every 24 h. (A1)
-
•
Blood investigations submitted for MELD score calculation should have been performed within 7 days (if MELD ≤30) or within 48 h (if MELD >30) of application. They should all be performedon the same day and must be from a single laboratory, which is either based at a licensed transplant hospital, government institute or a laboratory accredited by the National Accreditation Board for Testing & Calibration Laboratories. (B2)
-
•
Responsibility of regular updation revision of CSS remains with the listing unit transplant centre, and liver allocation can be done based on the updated score after 72 h from listing or last update. (B1)
Paediatric Liver Transplantation
LDLT is the primary means of LT for children in India due to low DD, acceptance of LDLT as a viable option by parents and the availability of technical expertise. Children are listed for DDLT in India only when the LDLT option is not available, and hence, it is recommended that a separate paediatric waiting list is maintained to ensure their access to paediatric livers or split liver grafts. Allocation of liver grafts for children should also be based on clinical urgency, and additional priority should be given to indications such as hepatoblastoma.20,21
A composite score (CSS-P) combining the PELD or MELD score (for children aged >12 years), cirrhosis complications and waiting time was developed based on the committee recommendations with the maximum score capped at 40 points (Table 5). Similar to adults, liver transplant indications where the PELD score does not predict actual wait-list mortality are allocated PELD exception points to improve their access to timely LT. In children, LT for unresectable hepatoblastoma despite neoadjuvant chemotherapy is associated with excellent long-term survival. However, these children have a small window of opportunity for LT after completion of the neoadjuvant chemotherapy and before disease progression. These children are hence allocated maximum exception points during wait listing. Similarly, certain noncirrhotic metabolic liver diseases in children such as urea cycle defects and organic acidemias can have frequent decompensation episodes leading to permanent neurological damage. These children are allocated the next tier of exception points in the CSS-P.
Table 5.
Proposed Clinical Severity Score-Paediatric (CSS-P). CSS-P score = MELD/PELD points + cirrhosis complications points + waiting time (maximum score is 40). PELD/MELD points = Aactual PELD∗ or MELD Score∗/2 (rounded off). Points capped at 20 (Use PELD for children <12 years, MELD for children 13–18 years).
| Cirrhosis complications points – Maximum of three complications can be considered | |
|---|---|
| Complications | Points added |
| Variceal bleeding | 5 |
| Moderate HPS (PaO2 <80 mm Hg and >60 mm Hg on room air) | 5 |
| Severe HPS (PaO2 <60 mm Hg on room air) | 10 |
| QOL issues (severe pruritus, frequent hospitalisation, etc) | 5 |
| Exception indications (Automatic allocation of PELD/MELD points) | |
|---|---|
| Indication | Points allocated |
| Hepatoblastoma | 40 |
| Noncirrhotic metabolic liver disease | 20 |
| Non-hepatoblastoma liver tumours | 15 |
| Abernathy syndrome | 15 |
| Multiorgan transplant | 15 |
| Waiting time points | |||||
|---|---|---|---|---|---|
| Waiting time (months) | 0–3 | 3–6 | 6–9 | 9–12 | >12 |
| Points | 0 | 2 | 5 | 7 | 10 |
HPS, hepatopulmonary syndrome; MELD, Model for End-Stage Liver Disease; PELD, Paediatric Model for End-Stage Liver Disease; QOL, Quality of life.
∗All reports used for PELD/MELD score calculation should have been performed within 7 days of updating, performed on the same day (or within 48 h of each other) and must be from a single laboratory, which is either part of a licensed transplant hospital, government institute or is an NABL-accredited laboratory.
The definition of paediatric deceased donors was discussed to identify the group of DDLG, which should be preferentially allocated to children. Various allocation systems across the world have differing age- and weight-based criteria.22,5 An audit of paediatric liver transplants performed at two centres in Tamil Nadu with significant Pediatric Liver Transplantation (PLT) activity showed that more than 50% of PLT recipients are aged less than 5 years and 90% of PLT recipients were younger than 10 years. (Personal communication GN,MSR) Studies have shown that the weight of the liver in a child of 10 years will be around 700 g.23 Livers larger than 700 g would not be suitable for most paediatric recipients unless they undergo further graft reduction. Hence, the sub-committee recommended that an age cut-off of 10 years be used to define paediatric liver donor. This would ensure that children on waiting list will receive maximum benefit from size-matched livers while livers from deceased donors over 10 years of age can still be used for small adults.
Most children needing LT are under 5 years of age and are suitable for a split left lateral segment graft transplant. Split Liver Transplantation (SLT) is still uncommon in India due to problems such as the logistic difficulties of organising the split procedure and lack of clear split graft sharing policies.24 Once a national system of liver allocation and sharing between centres is established, a national split policy should be developed to improve access of children to split liver grafts.
-
•
Separate waiting list for paediatric recipients (age <18 years) should be maintained. (B1)
-
•
Prioritisation of paediatric recipients should be based on CSS calculated using PELD/MELD score, associated complications of CLD and waiting time as criteria. (A1)
-
•
Indications not associated with high PELD despite high WL mortality such as unresectable hepatoblastoma or noncirrhotic metabolic liver diseases should be allocated exception points to improve their access to DDLT. (A1)
-
•
Cut-off age for defining paediatric deceased donors for the purpose of liver allocation is 10 years. (C2)
-
•
Liver grafts of paediatric deceased donors should be preferentially allocated to paediatric recipients before being offered to adult recipients. (B1)
-
•
A national split liver policy should be developed to improve access to organs for paediatric wait-listed recipients (B1)
Dealing with ties in Clinical Severity scores
It is likely that instances would arise where more than one patient may have the same CSS at the time of organ availability. In most cases, the rule of priority to the in-house recipient will guide organ allocation. When none of the competing patients are in-house recipients, then allocation should be according to the following rules.
When two or more adult patients have the same CSSs at the time of organ offer, priority will be given to the patient with a higher MELD score, acute decompensation score, cirrhosis complications score and, finally, actual waiting time in that sequence.
When two or more paediatric patients have the same CSSs at the time of organ offer, priority will be given to the patient with a higher PELD/MELD score and actual waiting time in that sequence.
When an adult and a child have the same CSSs at the time of organ offer, priority will be given to the patient with a longer waiting time in the case of an adult donor, while the child will be prioritised in the case of a paediatric donor.
-
•
When two or more adult patients within a region have the same CSS, prioritisation will be based on MELD score, acute decompensation score, cirrhosis complication score and actual waiting time in that order. (C1)
-
•
When two or more paediatric patients have the same CSSs at the time of organ offer, priority will be given to the patient with a higher PELD/MELD score and actual waiting time in that sequence. (A1)
-
•
When an adult and a child have the same CSSs at the time of organ offer from an adult deceased donor, priority will be given to the patient with a longer waiting time. (C2)
National listing policy
Most committee members agreed that there should be no barriers for patient movement across the country for transplantation. Patients can be listed in any Indian centre if they are citizens of India or have Overseas Citizenship of India and currently residing in India. Although some experts felt that this would unfairly disadvantage patients who cannot afford to travel to other states for listing, the counter-argument is that the existing imbalance in DD rates across the country means that not allowing patients to travel for DDLT would be inherently disadvantageous to patients from states with low DD rates.
Currently, a patient is allowed to be listed in only one transplant centre in a state. The committee agreed that multiple listings within one state should not be allowed. However, considering a recent draft proposal to restrict the listing of a patient for DDLT to one centre across the country, this recommendation may need modification.
-
•
All Indian citizens and Indian residents with Overseas Citizen of India status are eligible for DDLT listing in any state of their choice. (B1)
-
•
Allocation of organs should be based on clinical need and not on their nativity or state of Domicile. (A1)
-
•
An individual patient can only be listed for DDLT in one transplant centre per state. (C2)
Patient assessment for DDLT
LT is a major surgical undertaking, and a detailed assessment of the patient is necessary to assess the risk-benefit of this surgery.10 Each LT centre should have a protocol for LT assessment by a multidisciplinary team. Laboratory and radiological investigations should be supplemented by first-hand assessment of the patient by the LT team. Each potential recipient should have formal consultations with senior members of the liver transplant team – including surgeons, hepatologists and anaesthetists who will be involved in his/her perioperative care. This is an important measure to build patient confidence and trust, as admission for DDLT may happen after-hours and the patient may not be able to meet the core team members immediately before operation. Although this is the current practice in most centres, there is anecdotal evidence of patients being listed for DDLT by centres based on external reports alone, without actually seeing the patient. The committee felt that this would risk incomplete and suboptimal assessment, potentially jeopardising patient safety.
The committee agreed that while the broad lines of recipient evaluation should be uniform across the country, each centre may choose its own panel of specific investigations based on local expertise. Table 6 provides a list of suggested investigations and consultations before listing for DDLT.
-
•
Patients evaluated for DDLT should have a multidisciplinary assessment to evaluate their suitability for LT. (A1)
-
•
Each transplant unit should have an in-house liver transplant assessment protocol, including blood investigations, cross-sectional imaging, cardiorespiratory assessment and relevant speciality consultations. (A1)
-
•
All patients should have at least one prelisting consultation with the core transplant team likely to be involved in the peritransplant care of the patient (surgeon, hepatologist and anaesthetist). (A1)
-
•
Listing of a patient based on video consultations or review of investigations alone should not be done. (A1)
Table 6.
Suggested Protocol for Initial Evaluation and Follow-Up on Waiting List.
| Investigation | At assessment | On waiting list |
|---|---|---|
| Clinical assessment | Yes | MELD >30: Weekly MELD 20–30: Monthly MELD <20: Three monthly |
| Haematology, biochemistry, microbiology | Yes | MELD >30: Weekly MELD 20–30: Monthly MELD <20: Three monthly |
| Blood grouping | Yes | No |
| Infection screening | Yes | If indicated |
| Metabolic profile | Yes | If indicated |
| ABG as screening for HPS | Yes | If indicated |
| Cardiac assessment | Yes | At least 6 monthly or more frequent if indicated |
| Tumour markers | Yes | At least 6 monthly or more frequent if indicated |
| Cross-sectional imaging (Triphasic CT/MRI) | Yes | 3 monthly for patients with HCC 6 monthly for others |
| Thrombophilia assessment | Yes, if indicated | No |
| Tests for etiological diagnosis | Yes | No |
| Surveillance endoscopy | Yes | At least 6 monthly or more frequent if indicated |
| Gynaecology assessment for women | Yes | If indicated |
| Nutritional and functional assessment | Yes | At least 6 monthly or more frequent if indicated |
| Psychiatry assessment | Yes | If indicated |
CT, computed tomography; HCC, hepatocellular carcinoma; HPS, hepatopulmonary syndrome; MELD, Model for End-Stage Liver Disease; MRI, magnetic resonance imaging; ABG, Arterial blood gas.
Care of the wait-listed patient
Patients on the DDLT waiting list need close monitoring to detect changes in clinical condition, including acute deterioration, infection and worsening renal function. As the risk of acute deterioration increases with increasing initial clinical severity, frequency of follow-up should be based on the most recent MELD score.11,12 In addition, patients on waiting list also need monitoring for HCC and changes in the cardiovascular status at regular intervals. Follow-up visits, including clinical review and blood tests, are best performed in the listing transplant centre especially in sicker patients or patients with high MELD score. When this is not logistically possible and a stable patient is being managed by their local physician, treatment should be coordinated with the transplant unit to ensure correct assessment of clinical urgency and/or transplant futility. This is particularly important in patients who need repeated admissions for blood transfusion or broad-spectrum antibiotics, as these have the potential to impact immediate posttransplant outcomes.
Transplant centres should report any significant improvement or deterioration in a patient's condition so that his/her CSS score is revised immediately. Occasionally, the patient's clinical condition can improve with treatment to the extent that he/she does not satisfy the minimal listing criteria any longer (ie, MELD <15 with no cirrhosis complications) or no exception indications and/or HCC completed treated with locoregional therapy such as ablation or surgery. Such patients should be suspended on the waiting list for 3 months to monitor the clinical status. If the patient remains stable during this period without any deterioration, he/she can be removed from the list. Patients should also be considered for delisting if the HCC progresses beyond listing criteria due to the development of macrovascular invasion or systemic metastases or if the patient becomes too sick, making the liver transplant procedure futile.
-
•
Patients on the waiting list should be monitored periodically to identify any deterioration or improvement in health condition, which might have an influence on their eligibility for DDLT, wait-listing status and risk associated with LT. (A1)
-
•
Frequency of follow-up on the waiting list should be based on the clinical condition of the patient at the time of listing or most recent evaluation. (A1)
DDLT in PuSHs
Most LT activity in India is currently based in private hospitals.6 PuSHs have a huge untapped potential to increase organ donation. However, there is widespread notion that liver transplant is primarily a treatment for the financially well-off using organs from their poorer counterparts. This has started to change in recent years due to continuous efforts by both governmental and nongovernmental agencies. The committee felt that actively encouraging and supporting LT programmes in PuSHs by prioritising organ allocation to patients listed in these hospitals will help in swaying the public opinion towards DD in general and LT in particular. This will have the twin benefits of increasing DD and encouraging consistent LT activity in PuSH. In line with the proposed hybrid system of organ allocation, any donation in a private sector hospital will be first allocated to an in-house recipient with highest CSS before being offered to the general pool of wait-listed patients. The committee believed felt that in the case of a DDLG from a PuSH, we should go further and allocate such livers to recipients listed in other PuSH within the state before they are offered to patients listed in private sector hospitals. Highlighting such instances of organ sharing between various PuSH will be viewed positively by the public and the media and potentially provide increased visibility for DD.
-
•
For the purpose of these guidelines, the term ‘Public sector hospitals’ should include all hospitals that are administered either directly by the Government (Central or State or Municipal Corporation) or are Government-aided. (C2)
-
•
Patient listing policies for DDLT will be uniform across all private sector and public sector hospitals. (B1)
-
•
Within the cohort of patients listed in PuSHs, the sequence of offering will be based on the proposed CSS. (B1)
-
•
Liver grafts from donors identified in PuSHs (as defined above) will be preferentially allocated to recipients listed in PuSHs in the respective state/region before they are offered to patients listed in private sector hospitals, with the exception of super-urgent listed patients who will receive priority across all hospitals in each state. (C2)
Marginal grafts – definitions, protocols, graft preservation
A DDLG can be designated as a marginal or extended criteria graft when LT is associated with an increased risk of graft failure or early or long-term complications25 (Table 7). Marginal grafts, including livers recovered from donation after cardiac death donors, have become a valuable means of increasing DDLT activity in Western countries.26, 27, 28 There has been a reluctance to use marginal liver grafts in India primarily due to concerns of primary nonfunction or early allograft dysfunction as options for urgent retransplant are limited, and any postoperative complications can lead to spiralling cost of treatment. Utilisation of these organs is likely to increase as units gain greater experience in managing early allograft dysfunction and with the availability of improved organ preservation technologies. The decision to accept or reject a marginal graft is based on multiple factors, including donor clinical history, graft quality, logistics, individual centre experience and condition of the recipient. The committee agreed that there was currently no need for a separate policy for the allocation of such organs. Each centre can decide to accept or reject a marginal graft based on local factors after having a discussion with the potential recipient regarding risks associated with both transplanting and not transplanting such an organ.
Table 7.
Proposed Criteria for Defining Marginal or Extended Criteria Donors (Modified From Vodkin et al.25).
| Factors associated with increased risk of graft failure |
|
|
|
|
|
|
|
|
|
|
|
|
| Increased risk of disease transmission |
|
|
|
|
|
|
|
ALT, alanine transaminase; AST, aspartate transaminase; ICU, intensive care unit; CDC, Center for communicable disease; CIT, Cold ischemia time; ULN, Upper limit of normal.
Static cold storage with organ preservation solutions is the standard technique used in India for liver graft preservation. There is accumulating evidence for the benefit of machine perfusion to preserve DDLG, particularly marginal grafts or when prolonged cold ischaemia time is expected such as long-distance transport or difficult recipient surgery.29 Availability of these technologies is still limited in India and is especially hampered by their high cost. The committee felt that until clear evidence of their benefit and cost-benefit analysis in the Indian setting is available, usage of these systems should only be considered for selected instances after the potential benefits and risks have been discussed with the recipient and clearly documented.
-
•
Marginal grafts or extended criteria grafts are liver grafts associated with an increased risk of graft failure or increased early or late posttransplant morbidity. (A1)
-
•
Marginal grafts should be offered to patients according to the CSS, and the transplant team should discuss the risks associated with the transplantation of marginal organs with recipients. (C1)
-
•
Alternate modes of preservation such as machine perfusion can be considered for marginal grafts or when expected cold ischaemia time is longer than 8–10 h or when difficult explant surgery is expected. (B1)
-
•
Transplant teams using alternate means of preservation should discuss the possible benefits and risks with recipients. (B1)
Audit, quality control and periodic review
Success of any new system of organ allocation is entirely dependent on its actual application at the level of the transplant centre. A system of audit should be put in place to monitor each centre's compliance with the listing, prioritisation and organ allocation rules. It is recommended that all such audits are conducted by an independent committee constituted by the appropriate transplant authority for each state.
The CSS was developed based on consensus recommendations of the National Liver Allocation Policy (N-LAP) expert committee based on their collective experience and an extensive literature review. Some of these recommendations may not have robust supporting evidence yet in the Indian setting. It is suggested that these scores will be prospectively studied in a multicentre study to validate their relevance in predicting waiting list mortality. Data obtained through such an exercise can be used to fine-tune the system of prioritisation.
Finally, as the DDLT scenario in India matures, these N-LAP recommendations (Table 8) will need periodic review using evidence from accrued data to confirm that it serves the stated goal of a fair and just allocation of DDLG. It is suggested that the proposed policy undergoes a detailed review at least once every 2 years initially so that any identified problems can be resolved.
-
•
Compliance with the listing, prioritisation and allocation rules should be monitored by regular audits of transplant units conducted by an independent committee. (B1)
-
•
Periodic review of N-LAP should be conducted to ensure that the proposed policy remains fit for its intended purpose. (A1)
Table 8.
Final List of Recommendations With the Strength of Recommendations. (GRADE System-Level of Evidence: A, High Quality; B, Moderate Quality; C, Low Quality. Strength of Recommendation: 1, Good Consensus; 2, Moderate Consensus).
| S. Number | Recommendation | Level of evidence | Level of agreement |
|---|---|---|---|
| 1 | General principles | ||
| a | A nationally uniform system of wait-list prioritisation and allocation for deceased donor liver transplantation (DDLT) is necessary | A | 1 |
| b | Deceased donor liver graft (DDLG) allocation should be based on clinical urgency of patients on the waiting list | A | 1 |
| c | In the current Indian scenario where deceased donation is largely driven by individual transplant centres, a hybrid system of liver graft allocation has the best chance of successful application. | B | 1 |
| d | A hybrid system requires that a DDLG is first allocated to in-house recipients of the donor hospital with greatest clinical urgency, followed by patients with the highest clinical urgency in the common waiting list of respective state/region | B | 1 |
| 2 | DDLT indications and minimal listing criteria | ||
| a | Adult patients wait-listed for DDLT for decompensated cirrhosis should have a laboratory MELD score equal to or greater than 15 unless they fulfil other indications for liver transplantation | A | 1 |
| b | Patients with hepatocellular carcinoma within UCSF liver transplant criteria and unsuitable for curative therapies such as resection should be considered for DDLT listing irrespective of MELD score | A | 1 |
| c | Patients listed for acute liver failure will continue to be listed according to the modified NHSBT criteria for super-urgent liver transplantation listing | A | 1 |
| d | Patients with acute on chronic liver failure who need early LT should be considered for standard DDLT listing with additional priority points based on the number of failing organs or for living donor liver transplantation | B | 1 |
| 3 | Clinical severity score | ||
| a | Prioritisation on waiting list should be made using a composite clinical severity score with weightage given to MELD score, cirrhosis-related complications, HCC, number of organ failures and waiting time | B | 1 |
| b | For indications where MELD score does not reflect true clinical urgency, exception points will be allocated for each such diagnosis | B | 1 |
| c | Waiting time points will help ensure that patients with MELD scores in the intermediate range are not excessively disadvantaged by the ‘sickest first’ policy | C | 2 |
| d | Waiting time points will accrue after an initial waiting time of 3 months and capped beyond 12 months | C | 2 |
| e | Patients with HCC within UCSF criteria will be given additional points if the tumours are multifocal or at least one lesion is larger than 3 cm in diameter | B | 2 |
| 4 | Frequency of clinical severity score recalculation | ||
| a | Patients on the waiting list should have their MELD score recalculated at regular intervals to adjust the clinical severity score. Frequency of recalculation should be based on the most recent MELD score for that patient. Minimum frequency of testing is MELD >30 weekly, 20–30, monthly, and <20 every 3 months | B | 2 |
| b | Additional acute decompensation points given to patients listed on the elective DDLT waiting list should be renewed at least weekly, providing evidence of continued need for organ support and pertinent blood investigations performed within 24 h of application for renewal | B | 1 |
| c | Patients wait-listed for super-urgent DDLT should have their status renewed every 24 h | A | 1 |
| d | Blood investigations submitted for MELD score calculation should have been performed within 7 days (if MELD ≤30) or within 48 h (if MELD >30) of application. They should all be performed on the same day and must be from a single laboratory, which is either based at a licensed transplant hospital, government institute or accredited by the National Accreditation Board for Testing & Calibration Laboratories (NABL) | B | 2 |
| e | Responsibility of regular revision of CSS remains with the transplant centre and liver allocation can be done based on the updated score after 72 h from listing or last update | B | 1 |
| 5 | Paediatric liver transplantation | ||
| a | Separate waiting list for paediatric recipients (age <18 years) should be maintained | B | 1 |
| b | Prioritisation of paediatric recipients should be based on clinical severity score calculated using PELD/MELD score, associated complications of chronic liver disease and waiting time as criteria | A | 1 |
| c | Indications not associated with high PELD despite high WL mortality such as unresectable hepatoblastoma or noncirrhotic metabolic liver diseases should be allocated exception points to improve their access to DDLT | A | 1 |
| d | Cut-off age for defining paediatric deceased donors for the purpose of liver allocation is 10 years | C | 2 |
| e | Liver grafts of paediatric deceased donors should be preferentially allocated to paediatric recipients before being offered to adult recipients | B | 1 |
| f | A national split liver policy should be developed to improve access to organs for paediatric wait-listed recipients | B | 1 |
| 6 | Dealing with ties in CSS scores | ||
| a | When two or more adult patients within a region have the same clinical severity score, prioritisation will be based on MELD score, acute decompensation score, cirrhosis complication score, actual waiting time in that order | C | 1 |
| b | When two or more paediatric patients have the same clinical severity scores at the time of organ offer, priority will be given to the patient with a higher PELD/MELD score and actual waiting time in that sequence | A | 1 |
| c | When an adult and a child have the same clinical severity scores at the time of organ offer from an adult deceased donor, priority will be given to the patient with a longer waiting time | C | 2 |
| 7 | National listing policy | ||
| a | All Indian citizens and Indian residents with Overseas Citizen of India status are eligible for DDLT listing in any state of their choice | B | 1 |
| b | Allocation of organs should be based on clinical need and not on their nativity or state of Domicile | A | 1 |
| c | An individual patient can only be listed for DDLT in one transplant centre per state | C | 2 |
| 8 | Patient assessment for LT | ||
| a | Patients evaluated for DDLT should have a multidisciplinary assessment to evaluate their need and suitability for liver transplantation | A | 1 |
| b | Each transplant unit should have an in-house liver transplant assessment protocol including blood investigations, cross-sectional imaging, cardiorespiratory assessment and relevant speciality consultations | A | 1 |
| c | All patients should have at least one prelisting consultation with the core transplant team likely to be involved in the peritransplant care of the patient (surgeon, hepatologist and anaesthetist) | A | 1 |
| d | Listing of a patient based on video consultations alone or review of investigations alone should not be done | A | 1 |
| 9 | Care of the wait-listed patient | ||
| a | Patients on the waiting list should be monitored periodically to identify any deterioration or improvement in health condition, which might have an influence on their eligibility for DDLT, wait-listing status and risk associated with LT | A | 1 |
| b | Frequency of follow-up on the waiting list should be based on the clinical condition of the patient at the time of listing or most recent evaluation | A | 1 |
| 10 | DDLT in public sector hospitals | ||
| a | For the purpose of these guidelines, the term 'Public sector hospitals' should include all hospitals that are administered either directly by the Government (Central or State or Municipal Corporation) or are Government-aided | C | 2 |
| b | Patient listing policies for DDLT will be uniform across all private sector and public sector hospitals | B | 1 |
| c | Within the cohort of patients listed in public sector hospitals, the sequence of offering will be based on the proposed clinical severity score | B | 1 |
| d | Liver grafts from donors identified in public sector hospitals (as defined above) will be preferentially allocated to recipients listed in public sector hospitals in the respective state/region before they are offered to patients listed in private sector hospitals with the exception of super-urgent listed patients who will receive priority across all hospitals in each state | C | 2 |
| 11 | Marginal grafts and organ preservation | ||
| a | Marginal grafts or extended criteria grafts are liver grafts associated with an increased risk of graft failure or increased early or late posttransplant morbidity | A | 1 |
| b | Marginal grafts should be offered to patients according to the clinical severity score and the transplant team should discuss the risks associated with transplantation of marginal organs with recipients | C | 1 |
| c | Alternate modes of preservation such as machine perfusion can be considered for marginal grafts or when expected cold ischaemia time is longer than 8–10 h or when difficult explant surgery is expected | B | 1 |
| d | Transplant teams using alternate means of preservation should discuss the possible benefits and risks with recipients | B | 1 |
| 12 | Audit, quality control and periodic review | ||
| a | Compliance with the listing, prioritisation and allocation rules should be monitored by regular audits of transplant units conducted by an independent committee | B | 1 |
| b | Periodic review of N-LAP should be conducted to ensure that the proposed policy remains fit for its intended purpose | A | 1 |
CCS, Clinical Severity Score; DDLG, deceased donor liver grafts; DDLT, deceased donor liver transplantation; HCC, hepatocellular carcinoma; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; N-LAP, National Liver Allocation Policy; PELD, Paediatric Model for End-Stage Liver Disease; WL, Waiting list; NHSBT, National health service blood & transplant; UCSF, University of California San Francisco.
Areas for future research
The current consensus document includes several proposals, which are based on evidence from Western literature and expert consensus. The gradual increase in DDLT numbers in India is a potential opportunity for advancing research in the science of deceased donor organ allocation and waiting list management. Proposals such as the CSS, criteria for determining urgency and the impact of waiting time points should be validated in carefully planned Indian multicentre studies. Setting up of an Indian nationally uniform policy can help validate and/or fine-tune these protocols, which will become increasingly relevant to other middle-income countries looking to set up their own DDLT programs.
Credit authorship contribution statement
Mettu Srinivas Reddy: conceptualisation, project administration, supervision, methodology, data collection and analysis, writing, final approval.
Surendra Kumar Mathur: conceptualisation, project administration, supervision, methodology, data collection and analysis, writing, final approval.
S Sudhindran: conceptualisation, project administration, supervision, methodology, data collection and analysis, writing, final approval.
Subhash Gupta: methodology, supervision, data collection and analysis, writing, final approval.
Mohamed Rela: methodology, supervision, data collection and analysis, writing, final approval.
Arvinder Singh Soin: methodology, supervision, data collection and analysis, writing, final approval.
Darius Mirza: methodology, data collection & analysis, writing, final approval.
Sonal Asthana: methodology, data collection & analysis, writing, final approval.
Madhusudhan Chintakindi: methodology, data collection & analysis, writing, final approval.
Mathew Jacob: methodology, data collection & analysis, writing, final approval.
Vinay Kumaran: methodology, data collection & analysis, writing, final approval.
Pranjal Modi: methodology, data collection & analysis, writing, final approval.
Ravi Mohanka: methodology, data collection & analysis, writing, final approval.
Gomathy Narasimhan: methodology, data collection & analysis, writing, final approval.
Sujoy Pal: methodology, data collection & analysis, writing, final approval.
Viniyendra Pamecha: methodology, data collection & analysis, writing, final approval.
Amit Rastogi: methodology, data collection & analysis, writing, final approval.
Sanjiv Saigal: methodology, data collection & analysis, writing, final approval.
Manav Wadhawan: methodology, data collection & analysis, writing, final approval.
Conflicts of interest
All authors have none to declare.
Funding
None.
References
- 1.Kute V.B., Ramesh V., Rela M. On the way to self-sufficiency: improving deceased organ donation in India. Transplantation. 2021 Aug 1;105:1625–1630. doi: 10.1097/TP.0000000000003677. [DOI] [PubMed] [Google Scholar]
- 2.Jorm A.F. Using the Delphi expert consensus method in mental health research. Aust N Z J Psychiatry. 2015 Oct;49:887–897. doi: 10.1177/0004867415600891. [DOI] [PubMed] [Google Scholar]
- 3.Atkins D., Best D., Briss P.A., et al. Grading quality of evidence and strength of recommendations. BMJ. 2004 Jun 19;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Policy I of M (US) C on OP and T . National Academies Press (US); 1999. The ''Final Rule" Department of Health and Human Services 42 CFR Part 121 Organ Procurement and Transplantation Network; Final Rule (63 Federal Register 16295, at 16332, April 2, 1998)https://www.ncbi.nlm.nih.gov/books/NBK224637/ (Organ Procurement and Transplantation: Assessing Current Policies and the Potential Impact of the DHHS Final Rule). [Internet] [cited 2022 Aug 9]. Available from: [PubMed] [Google Scholar]
- 5.NHS Blood & Transplant . 2022. POL196/10.1 – Deceased Donor Liver Distribution and Allocation.https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/27193/pol195.pdf [Internet] [cited 2022 Aug 11]. Available from: [Google Scholar]
- 6.Nundy S. Why are so few liver transplants done in the public sector in India and how can we improve the numbers? J Clin Exp Hepatol. 2022 Aug;12:1029–1030. doi: 10.1016/j.jceh.2022.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanetto A., Shalaby S., Gambato M., et al. New indications for liver transplantation. J Clin Med. 2021 Aug 28;10:3867. doi: 10.3390/jcm10173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhary N.S., Saraf N., Saigal S., Soin A.S. Liver transplantation for acute on chronic liver failure. J Clin Exp Hepatol. 2017 Sep;7:247–252. doi: 10.1016/j.jceh.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarin S.K., Choudhury A., Sharma M.K., et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019 Jun 6;13:353–390. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin P., DiMartini A., Feng S., Brown R., Jr., Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 11.Samuel D., Coilly A. Management of patients with liver diseases on the waiting list for transplantation: a major impact to the success of liver transplantation. BMC Med. 2018 Aug 1;16:113. doi: 10.1186/s12916-018-1110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu. EASL Clinical Practice Guidelines: liver transplantation. J Hepatol. 2016 Feb;64:433–485. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Questions and answers about liver allocation - OPTN. https://optn.transplant.hrsa.gov/patients/by-organ/liver/questions-and-answers-about-liver-allocation/ [Internet]. [cited 2022 Aug 11]. Available from:
- 14.McCaughan G.W., Munn S.R. Liver transplantation in Australia and New Zealand. Liver Transpl. 2016 Jun;22:830–838. doi: 10.1002/lt.24446. [DOI] [PubMed] [Google Scholar]
- 15.Durand F. Development and outcomes of the French liver allocation system. Curr Opin Organ Transplant. 2020 Apr;25:132–138. doi: 10.1097/MOT.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 16.Sacleux S.C., Samuel D. A critical review of MELD as a reliable tool for transplant prioritization. Semin Liver Dis. 2019 Nov;39:403–413. doi: 10.1055/s-0039-1688750. [DOI] [PubMed] [Google Scholar]
- 17.Neuberger J. Allocation of donor livers--is MELD enough? Liver Transpl. 2004 Jul;10:908–910. doi: 10.1002/lt.20166. [DOI] [PubMed] [Google Scholar]
- 18.Bhangui P., Saigal S., Gautam D., et al. Incorporating tumor biology to predict hepatocellular carcinoma recurrence in patients undergoing living donor liver transplantation using expanded selection criteria. Liver Transpl. 2021 Feb;27:209–221. doi: 10.1002/lt.25956. [DOI] [PubMed] [Google Scholar]
- 19.Yoon Y.I., Lee S.G. Living donor liver transplantation for hepatocellular carcinoma: an Asian perspective. Dig Dis Sci. 2019 Apr;64:993–1000. doi: 10.1007/s10620-019-05551-4. [DOI] [PubMed] [Google Scholar]
- 20.NHS Blood & Transplant POL195/13 – Liver Transplantation: Selection Criteria and Recipient Registration. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/27193/pol195.pdf [Internet]. Available from:
- 21.NHS Blood & Transplant SOP5907/1 – Hepatoblastoma, Prioritised Paediatric and ACLF Registration Process. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/25018/sop5907.pdf [Internet]. Available from:
- 22.Kwong A.J., Ebel N.H., Kim W.R., et al. OPTN/SRTR 2020 Annual Data Report: Liver. Am J Transplant. 2022 Mar;22(suppl 2):204–309. doi: 10.1111/ajt.16978. [DOI] [PubMed] [Google Scholar]
- 23.Molina D.K., Pinneri K., Stash J.A., Li L., Vance K., Cross C. Organ weight reference ranges for ages 0 to 12 years. Am J Forensic Med Pathol. 2019 Dec;40:318–328. doi: 10.1097/PAF.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 24.Cherukuru R., Reddy M.S., Shanmugam N.P., et al. Feasibility and safety of split-liver transplantation in a nascent framework of deceased donation. Liver Transpl. 2019 Mar;25:450–458. doi: 10.1002/lt.25405. [DOI] [PubMed] [Google Scholar]
- 25.Vodkin I., Kuo A. Extended criteria donors in liver transplantation. Clin Liver Dis. 2017 May;21:289–301. doi: 10.1016/j.cld.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Guorgui J., Ito T., Younan S., et al. The utility of extended criteria donor livers in high acuity liver transplant recipients. Am Surg. 2021 Dec;87:1684–1689. doi: 10.1177/00031348211024658. [DOI] [PubMed] [Google Scholar]
- 27.Schlegel A., Foley D.P., Savier E., et al. Recommendations for donor and recipient selection and risk prediction: working group report from the ILTS consensus conference in DCD liver transplantation. Transplantation. 2021 Sep 1;105:1892–1903. doi: 10.1097/TP.0000000000003825. [DOI] [PubMed] [Google Scholar]
- 28.Laing R.W., Scalera I., Isaac J., et al. Liver transplantation using grafts from donors after circulatory death: a propensity score-matched study from a single center. Am J Transplant. 2016 Jun;16:1795–1804. doi: 10.1111/ajt.13699. [DOI] [PubMed] [Google Scholar]
- 29.Sousa Da Silva R.X., Weber A., Dutkowski P., Clavien P.A. Machine perfusion in liver transplantation. Hepatology. 2022 Apr;76:1531–1549. doi: 10.1002/hep.32546. [DOI] [PubMed] [Google Scholar]
- 30.Saraf V., Pande S., Gopalakrishnan U., et al. Acute liver failure due to zinc phosphide containing rodenticide poisoning: clinical features and prognostic indicators of need for liver transplantation. Indian J Gastroenterol. 2015 Jul;34:325–329. doi: 10.1007/s12664-015-0583-2. [DOI] [PubMed] [Google Scholar]
- 31.Reddy M.S., Rajakumar A., Mathew J.S., et al. Liver Transplantation Society of India guidelines for the management of acute liver injury secondary to yellow phosphorus-containing rodenticide poisoning using the modified Delphi technique of consensus development. J Clin Exp Hepatol. 2021 Aug;11:475–483. doi: 10.1016/j.jceh.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreau R., Jalan R., Gines P., et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013 Jun;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. 1437.e1–e9. [DOI] [PubMed] [Google Scholar]