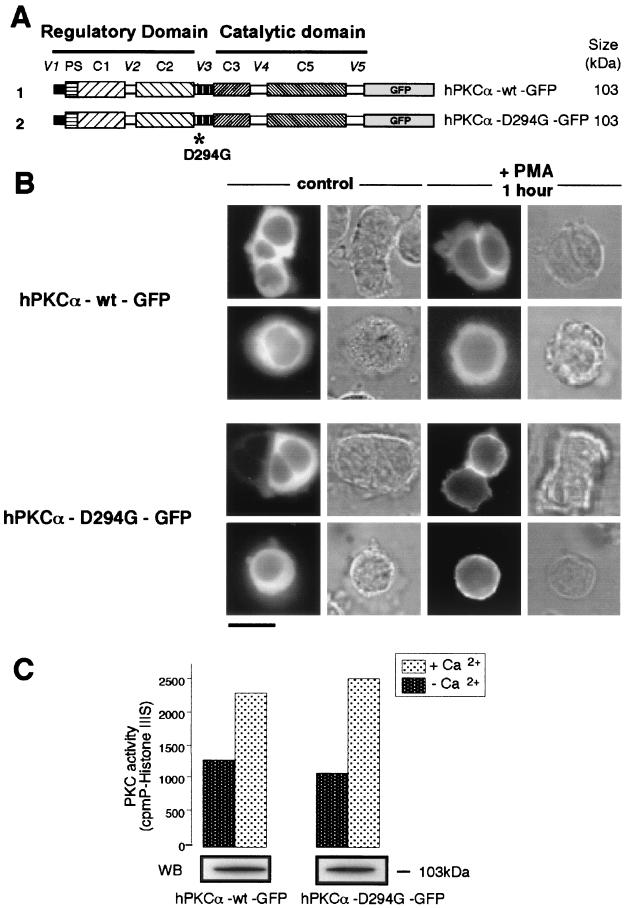
FIG. 1.
The presence of the natural D294G mutation abolishes the specific targeting of hPKCα at the interface between apposed cells upon PMA stimulation. (A) Schematic representation of hPKCα-wt–GFP and hPKCα-D294G–GFP fusion proteins with their expected molecular weights. As described in Materials and Methods, hPKCα-wt and hPKCα-D294G cDNA were subcloned in frame at the 5′ end of the sequence encoding GFP with EcoRI and KpnI sites. The D294G point mutation is localized in the V3 hinge region of hPKCα. (B) Localization of hPKCα-wt–GFP (top) and hPKCα-D294G–GFP (bottom) observed in transiently transfected living GH3B6 cells. In basal conditions, hPKCα-wt–GFP and hPKCα-D294G–GFP are both cytoplasmic. When cells are treated with 100 nM PMA for 1 h, hPKCα-wt–GFP is exclusively targeted at the interface between apposed cells. In contrast, in the same conditions, hPKCα-D294G–GFP is uniformly targeted at the plasma membrane of apposed cells and of isolated cells. Bar, 5 μm. (C) The GFP tag does not affect the activity of hPKCα-D294G. PKC catalytic activity was assessed by measuring the incorporation of 32P from [γ-32P]ATP into histone IIIS substrate in the presence of 10 μg of phosphatidylserine per ml and 10 μM PMA. The experiment was performed in the presence or in the absence of 1.2 mM calcium. Results show that histone IIIS is equally phosphorylated by hPKCα-wt–GFP and hPKCα-D294G–GFP. Western blot analysis of immunoprecipitated hPKCα-wt–GFP and hPKCα-D294G–GFP (revealed with the anti-GFP antibody) indicates the amount of each protein used for PKC activity assay. Three separate experiments gave identical results.