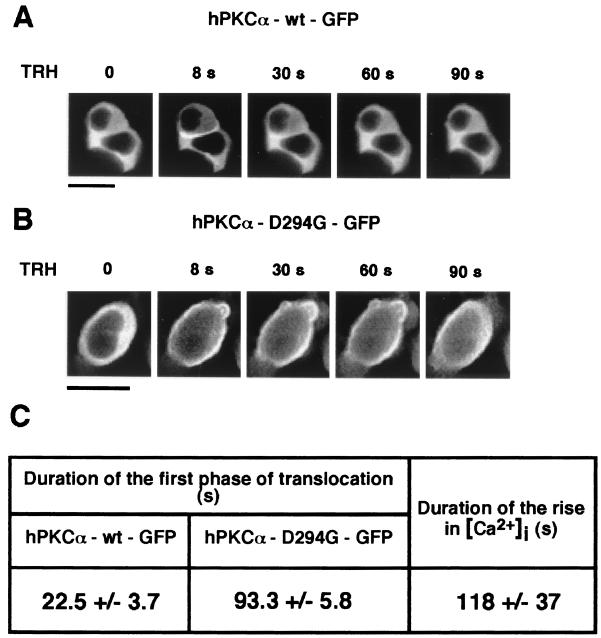
FIG. 2.
Time course of plasma membrane translocation of hPKCα-wt–GFP and hPKCα-D294G–GFP upon TRH stimulation. (A and B) GH3B6 cells expressing hPKCα-wt–GFP (A) or hPKCα-D294G–GFP (B) were observed with a confocal microscope immediately before and during stimulation with 100 nM TRH. Images were recorded every 2 s for 3 min. Translocation was observed for the two proteins as soon as 8 s after the beginning of stimulation. As upon PMA stimulation, hPKCα-D294G–GFP lost the selective targeting to the cell-cell contacts. Indeed, as observed in this single cell (B), hPKCα-D294G–GFP translocated uniformly at the plasma membrane. Although TRH-induced translocation was reversible for both proteins, hPKCα-D294G–GFP remained at the plasma membrane for a longer time than the wild-type enzyme. Bar, 5 μm. (C) Duration of the translocation of hPKCα-wt–GFP and hPKCα-D294G–GFP and of the increase in [Ca2+]i induced by TRH stimulation. The cytosolic variations in [Ca2+]i were recorded using real-time scanning laser confocal imaging. Cells were loaded with 50 μM Fluo-3/AM for 30 min at 37°C, and the variation in F/Fmin values was calculated from the recorded fluorescence of the cells. Values are given as the mean ± the standard deviation. Duration of wild-type enzyme translocation is statistically different from that of the mutant (P < 0.005) and different from duration of [Ca2+]i increase (P < 0.0002). The duration of mutant translocation is not statistically different from duration of the [Ca2+]i rise (P > 0.8).