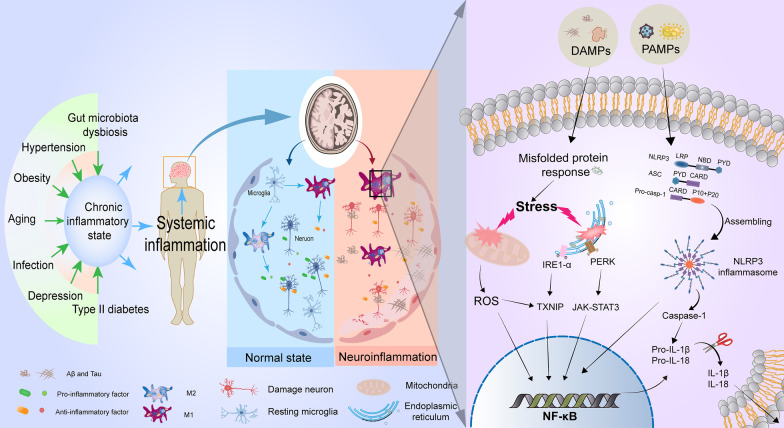
Fig. 1.
The molecular regulation of neuroinflammation for AD. At the early stage of AD, the body presents a chronic low-inflammatory state induced by aging, hypertension, type II diabetes, obesity, infection, and other risk factors, which can release a variety of danger-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) to activate immune responses of the nervous system. Resting microglia can be converted into a pro-inflammatory M1 phenotype to clear these danger signals for returning to a resting state. With the progression of the disease, under the continuous stimulation of DAMPs dominated by hyperphosphorylated Tau protein, extensive endoplasmic reticulum stress, oxidative stress, and the formation of NOD-like receptor protein 3 (NLRP3) inflammatory cells after assembly by NLRP3 in activated microglia are triggered, which promotes the entry of nuclear factor kappa-B (NF-κB) into the nucleus, thus resulting in the up-regulation of inflammatory genes (such as IL-1β and IL-18) to restore neural homeostasis. Thus, the accumulation of mis-folded proteins, M1-phenotype microglia, and inflammatory factors contribute to the neuroinflammatory microenvironment. Neuroinflammation also promotes the diffusion of hyperphosphorylated Tau protein in the brain, thereby creating a positive feedback loop to drive AD