Fig. 3 |. Mechanisms of endothelial cell senescence induced by damaging stimuli.
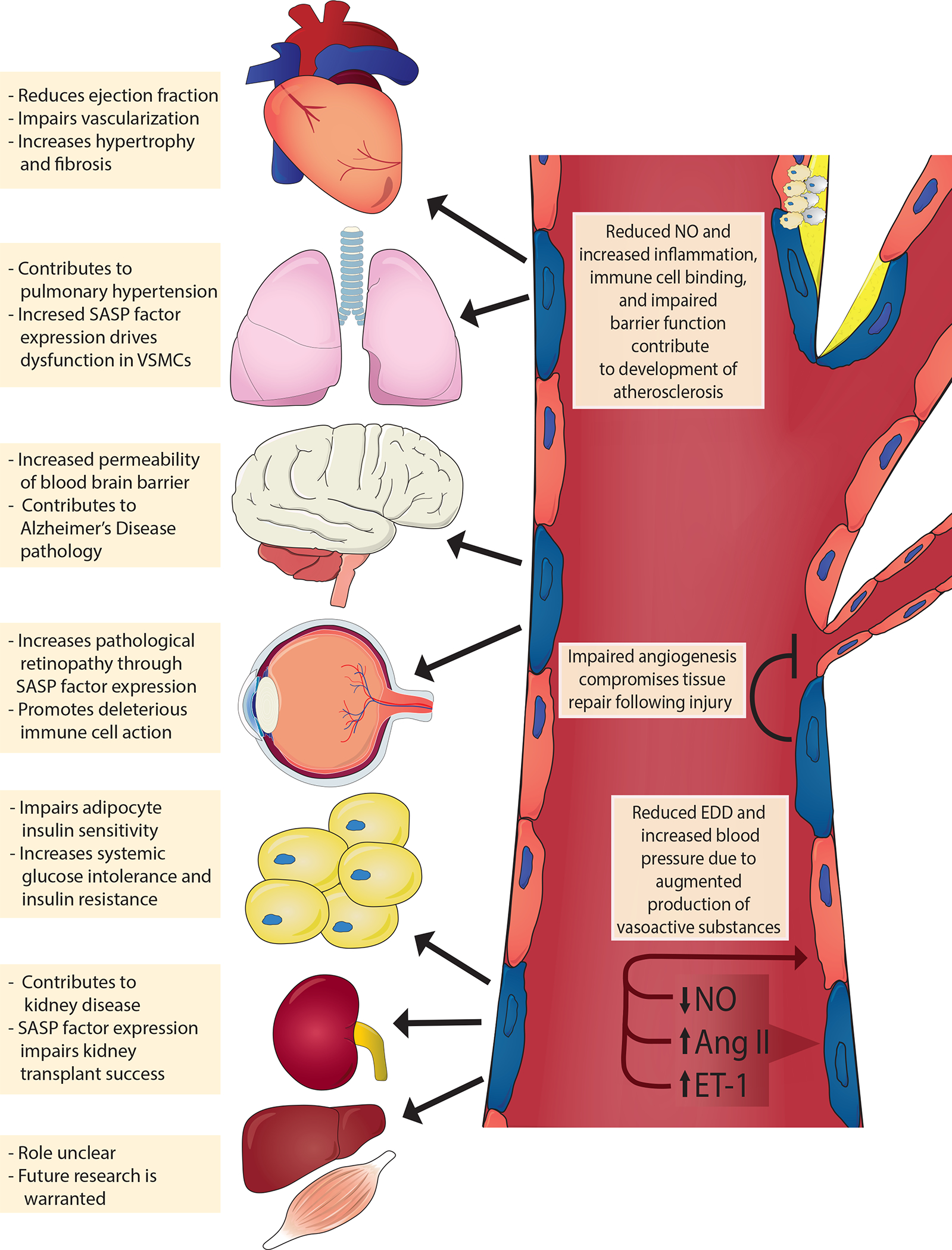
Circulating and endogenous stimuli result in telomeric and non-telomeric DNA damage and alter energy sensor pathways, such as those involving the sirtuins (SIRT1, SIRT3 and SIRT6). Consequently, endothelial cells activate tumour suppressor pathways (tumour antigen p53–cyclin-dependent kinase inhibitor 1A (also known as p21) and retinoblastoma protein (pRb)–cyclin-dependent kinase inhibitor 2A (also known as p16), which reprogrammes endothelial gene expression resulting in a host of cellular and molecular changes. For example, endothelial cells lose their proliferative potential and become senescent (blue cells); activate the senescence-associated secretory phenotype (SASP), which includes inflammatory pathways such as nuclear factor NF-kappa B (NF-κB) and secretion of inflammatory cytokines and reactive oxygen species (ROS); and attenuate the production of critical vasoactive molecules (e.g. nitric oxide (NO)). Collectively, the cellular and molecular changes promote arterial dysfunction resulting in cardiometabolic diseases. CXCL11, C-X-C motif chemokine 11; DDR, DNA damage response; MMPs, matrix metalloproteinases; PAI-1, plasminogen activator inhibitor 1; TGF-β, transforming growth factor-β.
