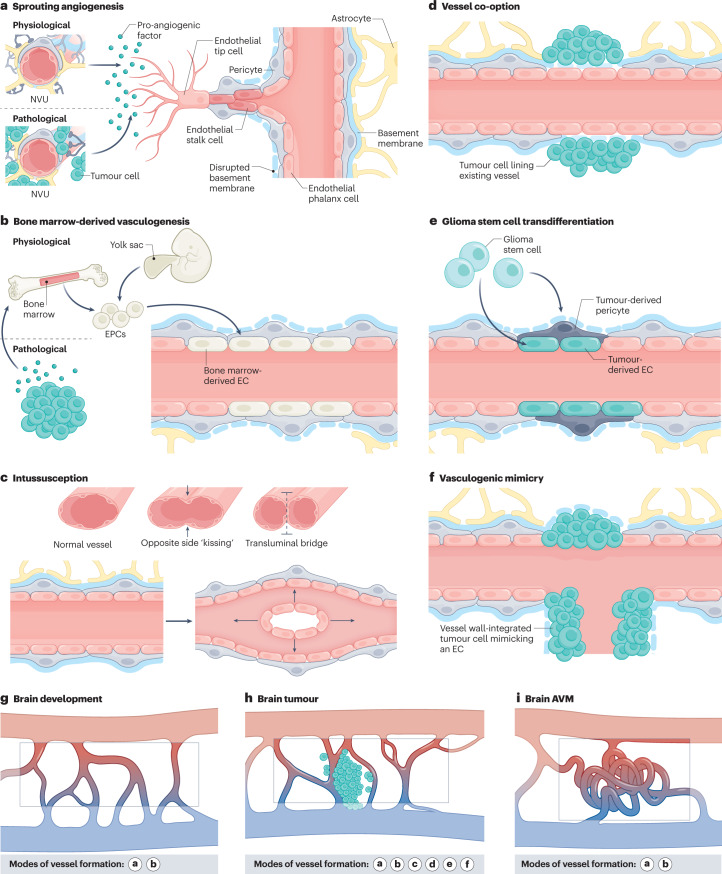
Fig. 1. Modes of vessel formation during brain development, in brain tumours and in brain AVMs.
Vascularization during brain development, in brain tumours and in brain arteriovenous malformations (AVMs) can occur via different modes of neovascularization. a, Neovascularization is possible via the formation of new blood vessels from pre-existing ones in response to pro-angiogenic signalling molecules secreted by components of the neurovascular unit (NVU) (defined as physiological sprouting angiogenesis) or by tumour cells (defined as pathological sprouting angiogenesis). For simplicity, the NVU (in physiological conditions) and tumour cells (in pathological conditions) are illustrated as sources of pro-angiogenic molecules for this mode of neovascularization. Note that the secretion of pro-angiogenic molecules is not limited to these sources but can also occur from brain vascular malformations and other vascular-dependent brain pathologies as well as from components of the extracellular matrix. New vessel sprouts are guided by specialized endothelial tip cells extending multiple filopodial protrusions sensing and reacting to pro-angiogenic, anti-angiogenic and hypoxia-related cues in the microenvironment. At the back of the leading tip cell, proliferating endothelial stalk cells elongate the growing blood vessel and initiate the formation of a functional lumen. Phalanx cells are the most quiescent of the endothelial cell (EC) subtypes, extend few filopodia and migrate and divide poorly in response to VEGF. Endothelial phalanx cells line vessels once the new vessel branches have been consolidated. b, Physiological vasculogenesis is defined as the de novo generation of blood vessels from either yolk sac-derived endothelial progenitor cells (EPCs) or bone marrow-derived EPCs, depending on the developmental time point. Pathological vasculogenesis occurs upon secretion of pro-angiogenic molecules by tumour cells that activate bone marrow to produce EPCs. Both indirect paracrine secretion of pro-angiogenic growth factors and direct luminal incorporation into sprouting nascent vessels contribute to vasculogenesis. Note that the secretion of pro-angiogenic molecules is not limited to these sources but can also occur from brain vascular malformations and other vascular-dependent brain pathologies as well as from components of the extracellular matrix. c, The splitting of existing blood vessels — vascular intussusception — allows the reorganization of existing cells without a corresponding increase in EC number. During this process, the opposite capillary walls invaginate into the vessel lumen in consecutive steps with the formation of a transluminal bridge of pericytes, myofibroblasts and extracellular matrix. d–f, Pathological conditions such as tumours or regenerative processes can exhibit the aforementioned modes of vessel formation and three additional ones, namely vessel co-option, glioma stem cell to EC transdifferentiation or glioma stem cell to pericyte transdifferentiation, and vasculogenic mimicry. Vessel co-option occurs when tumour cells co-opt existing vessels in response to angiopoietin 2 (ANG2) expression gradients (part d). In glioma stem cell transdifferentiation, glioma stem-like cells differentiate into either tumour-derived ECs or tumour-derived pericytes, induced predominantly by the TGFβ and NOTCH1 pathways in hypoxic conditions (part e). In vasculogenic mimicry, tumour cells (instead of ECs) are incorporated into the inner vessel wall, forming functional vessel-like structures and thereby mimicking ECs (part f). g–i, Modes of vessel formation involved in angiogenesis during brain development (part g), in brain tumours (part h) and in brain AVMs (part i).