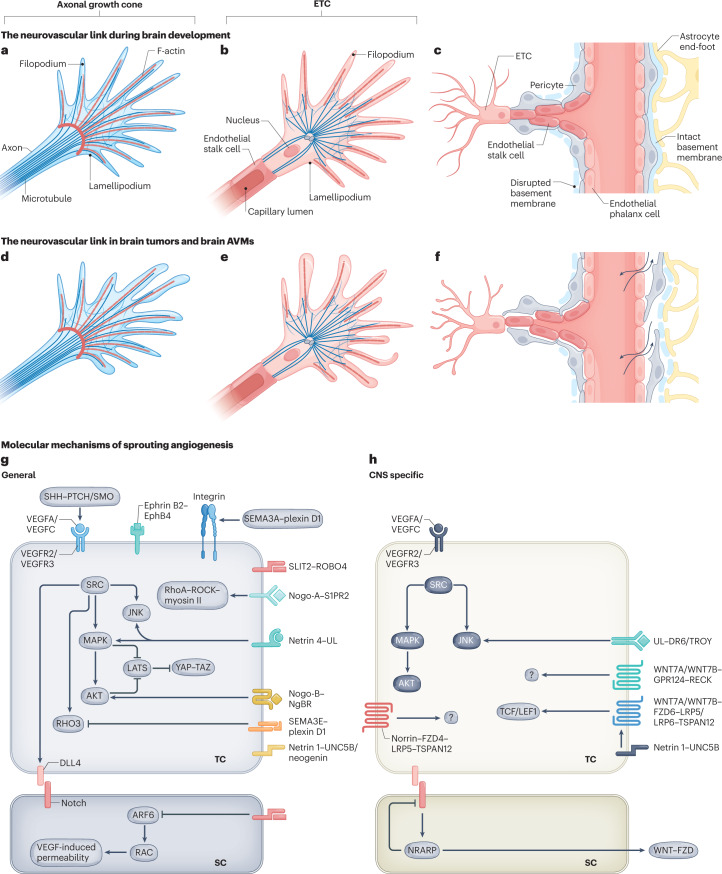
Fig. 2. Neurovascular link molecules affecting endothelial tip cell sprouting during vascular brain development, in brain tumours and in brain AVMs.
a, The axonal growth cone at the leading edge of a growing axon is a specialized, subcellular ‘hand-like’ structure at the tip of an extending neuron. In the axonal growth cone, lamellipodia and filopodia sense and integrate attractive and repulsive guidance cues in the local tissue microenvironment, thereby guiding the extending axon to its target. The central domain of an axonal growth cone is rich in microtubules, whereas the peripheral domain predominantly contains filopodia (composed of F-actin bundles) and lamellipodia (composed of an actin meshwork). Some microtubules extend into the peripheral domain and rarely into filopodia. b, The endothelial tip cell (ETC) is a specialized vascular endothelial cell type at the tip of the newly forming blood vessel, followed by proliferating endothelial stalk cells. Similarly to axonal growth cones, ETCs are specialized, ‘hand-like’ structures at the forefront of growing blood vessels that sense environmental cues using lamellipodia and ‘finger-like’ filopodia, thereby guiding the growing blood vessels to their respective targets. Endothelial phalanx cells comprise a third, mostly silent vascular endothelial cell type, lining the border of functional, established blood vessels (not shown). ETCs use actin-based lamellipodia and filopodia sensing attractive and repulsive guidance cues in the local tissue microenvironment to reach their target. Microtubules have not been detected in filopodia so far. c, A newly forming blood vessel sprout including a migrating ETC extending multiple filopodia, followed by proliferating endothelial stalk cells creating a newly formed capillary lumen, and quiescent endothelial phalanx cells lining an established vascular blood vessel. Pericytes, astrocytes and the basement membrane are also depicted. d–f, Schematic illustrations showing the characteristics of the axonal growth cone (part d), ETC (part e) and vessel sprouting (part f) in pathological conditions. Newly formed vessels often show a disrupted basement membrane, vascular leakage and a reduced pericyte coverage (part f). g,h, Molecularly, sprouting angiogenesis into the CNS is regulated by neurovascular link molecules that act in a non-CNS-specific way (part g), such as VEGFA–VEGFR2, SEMA3A/SEMA3E–plexin D1, ephrin B2–EphB4 and SLIT2–ROBO4, or a CNS-specific manner (part h), such as WNT7A/WNT7B–GPR124–FZD6–RECK and DR6–TROY. Of note, the VEGFA/VEGFC–VEGFR2/VEGFR3 and netrin 1–UNC5B signalling axes are shown in part h because even though they represent non-CNS-specific mechanisms, multiple CNS-specific mechanisms interact with these pathways downstream. AVM, arteriovenous malformation; SC, stalk cell; TC, tip cell; UL, unknown ligand.