ABSTRACT
Probiotics are used for both generally healthy consumers and in clinical settings. However, theoretical and proven adverse events from probiotic consumption exist. New probiotic strains and products, as well as expanding use of probiotics into vulnerable populations, warrants concise, and actionable recommendations on how to work toward their safe and effective use. The International Scientific Association for Probiotics and Prebiotics convened a meeting to discuss and produce evidence-based recommendations on potential acute and long-term risks, risks to vulnerable populations, the importance for probiotic product quality to match the needs of vulnerable populations, and the need for adverse event reporting related to probiotic use. The importance of whole genome sequencing, which enables determination of virulence, toxin, and antibiotic resistance genes, as well as clear assignment of species and strain identity, is emphasized. We present recommendations to guide the scientific and medical community on judging probiotic safety.
KEYWORDS: Probiotic, safety, next-generation probiotics, antibiotic resistance, microbiome, microbiota, genome sequencing, live biotherapeutic products, International Scientific Association for Probiotics and Prebiotics, ISAPP
Plain Language Summary
What is the context? Probiotics, available to healthy consumers as both dietary supplements and foods, are also used by some patient populations. The goal of this paper is to determine if any new factors have emerged that would impact current views about probiotic safety for both these populations.
What is new? The authors conclude that established practices are sensibly addressing factors important to the safety of traditional probiotics used by the general population. They also make recommendations regarding emerging safety considerations. Probiotics targeted for patient populations should undergo stringent testing to meet quality standards appropriate for that population, preferably verified by an independent third party. The safety of probiotics derived from species without a history of safe use must be considered on a case-by-case basis. Research is needed to address some gaps, for example which best animal models to use for safety assessment of live microbes, the possibility of antibiotic resistance gene transfer via transformation, and potential impact of probiotic-induced changes in microbiomes, interactions with drugs, and probiotic colonization.
What is the impact? Probiotics of sufficient quality for patient populations are being developed and should be used accordingly. Long-term safety assessments for probiotics should be consistent with, and not more stringent than, current regulatory requirements for biologic drugs, including fecal microbial transplants. Rigor in collecting and reporting data on adverse events is needed. The authors confirm the need for understanding the entire genetic makeup of a probiotic as a cornerstone for assessing its safety.
Background
Probiotics have been defined as ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’, a definition that was a grammatical edit of a previous FAO expert consultation.1 In the last 20 years, the number and quality of clinical trials assessing the health benefits of probiotics has grown substantially. Advances in efficacy measurement have been integral to the growth of the category. As is the case for any intervention, it is important not just to measure benefits but also to characterize risks. Early probiotics were associated with traditional uses in naturally fermented food products and thus not viewed as drugs, perhaps leading to less attention in earlier probiotic research to adverse event (AE) monitoring and reporting. Among the general population and in patients who are not immunocompromised or severely debilitated, acute safety issues appear to be minor, especially when considering the large global use of probiotics in foods and nutritional supplements.2–5 Further, more recent clinical trials reflect much improved reporting of AEs. As is the case with most interventions, though, longer-term safety endpoints are seldom tracked by trialists. As live products, there are theoretical risks of long-term impact on microbiota, immunity, cardiometabolic, and other physiological parameters that deserve further discussion.
The topic of probiotic safety has been addressed by several groups.6–10 A foundational initiative by the European Food Safety Authority established the Qualified Presumption of Safety approach for live microorganisms used in foods.11 This guidance is useful for recognizing microbial species with a history of safe use in foods. Pariza and colleagues12 proposed a 15-step decision tree to guide safety evaluations for products that lack an established history of safe use. An important paper modeled how to evaluate safety at the strain level, applied to the widely used Enterococcus faecium SF68.13 Roe and colleagues14 summarized best practices for assessing the quality and safety of probiotics, noting diverse regulatory frameworks utilized in different global regions.
A highly cited and thorough review of probiotic safety was conducted by the Southern California Evidence-based Practice Center, sponsored by the Agency for Healthcare Research and Quality.5 The review conducted in 2009 identified 622 studies, of which only 235 (37%) provided nonspecific statements about safety. Hempel et al. concluded that, from the available evidence, although interventions and AEs were poorly documented, there was no statistically significant increase of the relative risk (RR) of the overall number of experienced AEs (RR 1.00; 95% confidence interval: 0.93, 1.07, p = 0.999) associated with probiotic use.
Over the 12 years since the Hempel and colleagues review was published, improved knowledge of the microbiota and probiotics warrants a new look at the question of probiotic safety. This paper is the result of a discussion of an expert panel convened by the International Scientific Association for Probiotics and Prebiotics (ISAPP) at their 2022 annual meeting. ISAPP is a non-profit organization dedicated to advancing the science of probiotics and prebiotics. The assembled panel considered emerging issues pertaining to the safety of probiotics that warrant reconsideration as new data arise (Figure 1). Such issues include potential acute and long-term risks, the need for long-term studies, considerations for vulnerable target populations, probiotic product quality, and the importance of robust reporting of adverse events. The authors met for a half-day to discuss information presented by some panelists leading to a general agreement on key conclusions and recommendations. Individuals authored sections related to their expertise and that text was compiled, reviewed, and edited by all authors.
Figure 1.
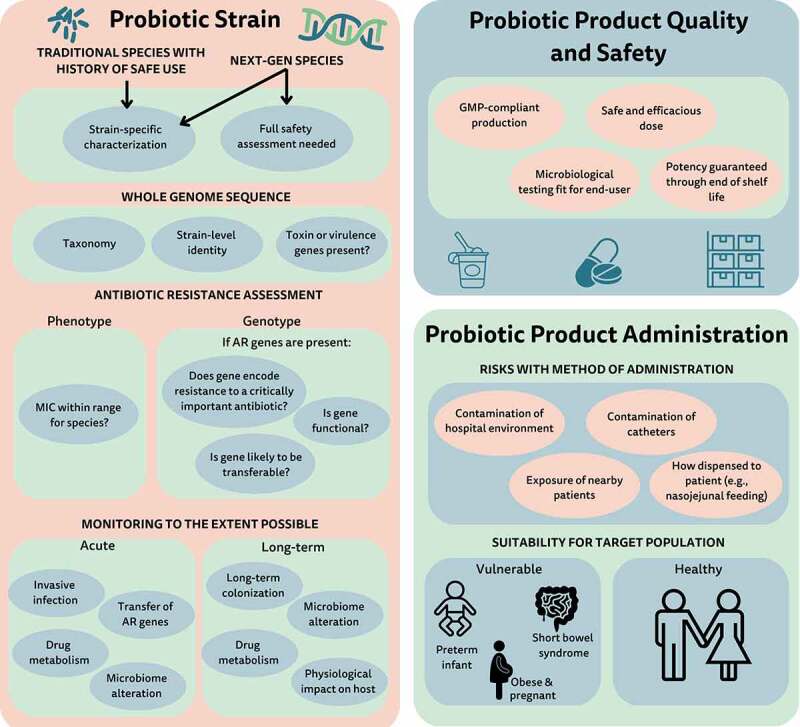
Factors for safety assessment of probiotics. AR, antibiotic resistance; GMP, good manufacturing requirements; MIC, minimum inhibitory concentration; Next-gen, next-generation.
Fundamentals of probiotic safety
Concerns related to the safety of probiotic organisms have been raised by clinicians, researchers, and policymakers.15–17 These may be broadly characterized into concerns pertaining to the probiotic strain, product quality, or probiotic administration.
A foundation to assessing the safety of any given probiotic strain is a complete genome sequence. This allows assignment of the strain to a given taxonomic group, enabling review of published risks associated with the species. A full genome sequence also allows strain-level identification, which can be important for tracking the strain during production and to investigate etiology of suspected infections. Further, the genome can be interrogated for any genes of concern, including toxigenicity, pathogenicity, or antibiotic resistance (AR). As discussed in more detail below, one concern is the theoretical situation in which probiotic-borne AR genes could be transferred to resident potential pathogens, other microbes harbored by the host, and/or environmental microbes, thereby increasing the ecological pool of antimicrobial resistance genes.15–17 Scientists are still evaluating the real transfer risk and the clinical and public health implications of such transfer. Some phenotypic testing is also a component of assessing safety of the probiotic strain.
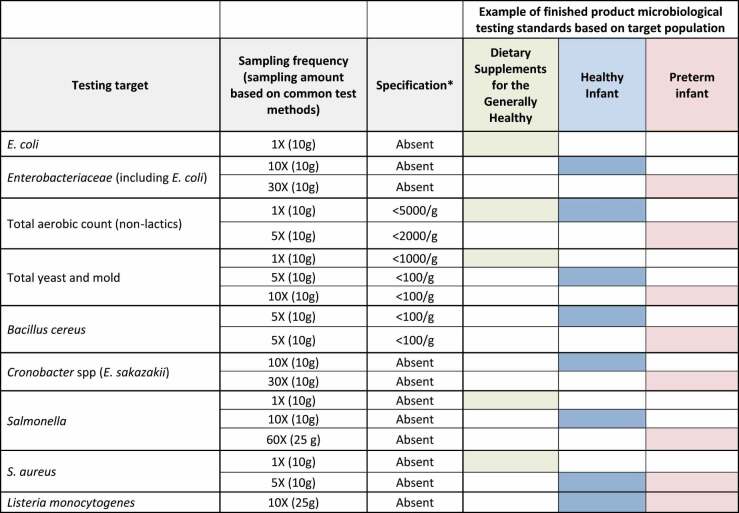
Issues related to safety pertaining to formulation of probiotic products include the need to establish purity, potency (the quantity of live microbes delivered), and composition of the final product. Further, probiotic products must undergo adequate testing – adapted to the intended use – for potential contaminants.15–17 Of particular concern is the presence of unwanted live microbial contaminants. Since probiotics are designed to be administered as live microbes, contamination with pathogenic or potentially pathogenic microbes is a greater risk than for products that undergo an intentional sterilization process. Testing specifications can be tailored for products, with products destined for more vulnerable populations undergoing testing that is more stringent than those used for the general population (Table 1). Microbial contamination of the final product and the presence of allergens or other contaminants are also concerns, but no more so for probiotics than for any other intervention.
Table 1.
Example testing requirements for a given probiotic developed for different product types and target populations. Note that stringent testing requirements can be imposed for products targeted for the most vulnerable populations.
 |
The manner in which the probiotic is given or taken must also be safe. This constitutes safe administration of a properly designed product to the intended host. Safe administration includes an appropriate route of administration to the host and correct manipulation or preparation of the probiotic on site. Products should be delivered at a dose and in a final formulation shown to be safe. Finally, the probiotic must be safe for the host, considering co-morbidities and underlying health concerns. These concerns extend beyond the proper formulation and production. For example, one such administration concern is the potential for cross-contamination of the hospital environment and of vulnerable patients once the probiotic is opened and used on site.18 Mixing a dried probiotic in hospital rooms has led to infection of intravenous catheters.19 The PROPATRIA study raised the concern that naso-jejunal administration may be contraindicated for some formulations administered to critically ill patients.20
Surveillance systems that facilitate both the reporting of AEs per doses administered and the removal from hospital formularies of probiotic product formulations that fail to fully identify the probiotic microorganism (genus, species, and strain) and potency through the end of product shelf-life would enhance the safety of probiotic consumption. In addition, education of providers of medical, nutrition, and healthcare information about the potential risks and benefits of the administration of a probiotic product in individual situations could facilitate shared decision-making regarding the use of probiotic products.17,21,22
Acute risks
Assessment of microbiota composition or function alterations
Microbiome profiling is the process of assessing baseline microbiota composition and community structure, as well as transcriptional and metabolic readouts. Assessing microbiome outcomes before and after probiotic intake may point to a causal role of probiotics in shaping the microbiome.23, 24 Such research may potentially inform hypotheses regarding microbial mechanisms and pathways that promote health, raise safety concerns or determine characteristics of responders and non-responders to probiotic therapy.24, 25 Such profiling may have a role in explaining or to a certain degree, predicting, the capacity of probiotics to confer beneficial effects on specific hosts.25
These microbiome profiling studies are only occasionally performed in probiotic research. For example, several profiling studies have revealed that intrinsic microbiome features determine probiotic engraftment in microbial communities of consumers.26 This suggests that microbiome assessment may provide a tool to tailor probiotic therapy and optimize clinical outcomes. This was observed in a study of Lacticaseibacillus rhamnosus GG supplementation of children with cystic fibrosis, where bifidobacteria-dominated fecal microbiota were associated with better clinical outcomes.27 In another study, probiotics promoted microbiota maturation, which was associated with reduced sepsis and inflammation in preterm infants.28, 29
An association between microbiome features and AEs has not been hitherto described, mostly owing to the paucity of research or inadequate harms reporting.30 We therefore recommend that microbiome profiling not be a required component of safety assessment for probiotics. However, such analysis is encouraged, as it may be useful for illuminating determinants of inter-individual differences in response to probiotics and for testing mechanisms and hypotheses.
Horizontal transfer of antibiotic resistance genes
Since the term probiotic entered the regulatory lexicon via the FAO/WHO expert consultation31 and guidelines,32 special attention has been paid by regulators, industry, and researchers to the risk of horizontal transfer of AR genes from probiotics to potential pathogens in the gut. In vivo transfer via conjugation of AR genes from viable probiotic bacteria acting as donors was documented in 199833 and several papers since have confirmed the transferability of AR genes within the gut for a recent review see.34 In general, attention is focused on critically important antibiotics as indicated by the World Health Organization.35, 36
Assessing the risk of existing AR genes within probiotic genomes requires both genotypic and phenotypic approaches. The phenotypic approach requires assessment of AR genes expressed by a strain as determined by minimum inhibitory concentration techniques.37–39 In some cases, the expression of an AR gene is intrinsic to the species and not due to the expression of genes that can be acquired.40, 41 The vancomycin “insensitivity” present in heterofermentative lactobacilli is an example of such intrinsic resistance.42 Such resistance is not a safety concern. However, the basis of any phenotypic resistance that is outside the norm for the species must be investigated further. Normal AR ranges for species of common probiotics have been established.37, 39, 43, 44 However, when developing strains of species for which such information is not known (next-generation strains), further study is required.
The genotypic approach requires complete genome sequencing (including plasmids) and identification of any known AR genes from the sequence. In general, if those genes are flanked by mobile elements or are plasmid-encoded, the strain should not be commercialized,12 but this prohibition may also depend on the antimicrobial in question, its current clinical utility and a risk/benefit analysis for the strain. When AR genes are located in the chromosome and do not have any genetic features that would suggest they are mobilizable, safety must still be carefully considered. If the resistance is for an antibiotic that is not deemed clinically relevant,35 it poses low risk. However, if the gene encodes non-intrinsic resistance to an antibiotic that is of clinical importance,35, 36 such as vancomycin for example, then the prudent approach would be to not commercialize that strain, unless further consideration determined that it was safe.
The regulatory approach to the tetracycline resistance gene, tet(W), in Bifidobacterium animalis subsp. lactis BB-12 is an example of such a case. The U.S. Food and Drug Administration (FDA), when informed of the presence of this gene, did not question the conclusion that its presence was neither clinically nor environmentally relevant and determined that it did not impact the strain’s status of generally recognized as safe for use in infant formula.45 This was based on the absence of a likely mechanism for transfer, the pervasiveness of tetracycline resistance among microbes in general, and the lack of clinical use of tetracycline in infants in the United States. Furthermore, in 2011 the European Food Safety Authority commented about the presence of the tet(W) gene in B. animalis subsp. lactis and concluded that there was no new information that would require a modification of the subspecies’ qualified presumption of safety status.11
Even in the absence of risk of conjugal transfer of non-intrinsic AR genes, there is also a possibility of transfer via transformation of naked DNA46, 47 or transduction via phage.48 Transformation is possible from DNA from non-viable cells, such as cells that have died during production or storage. This concern for dead microbes being a source of AR genes is reflected in the regulatory approach to safety for a postbiotic, which comprises non-viable but beneficial microbes.49 The European Food Safety Authority50 adopted a precautionary approach in the evaluation of a pasteurized strain of Akkermansia muciniphila as a novel food.51 The evaluation panel stated: “According to the applicant, the findings suggest that the strain does not harbor any AMR [antimicrobial resistance] genes of concern.” This was based on an in-silico interrogation of AR genes in the genome of the novel strain for AR genes included in the Comprehensive Antibiotic Resistance Database52 and National Database of Antibiotic Resistant Organisms.53
When considering the safety of AR genes in the genome of probiotics, several issues should be considered: (i) are the genes associated with genes that provide a likely mechanism for horizontal transfer; (ii) are the genes capable of functional expression if transferred to a naïve host; (iii) is the resistance phenotype for the antibiotic typical for the species (intrinsic resistance); (iv) is the resistance phenotype so widespread that the probiotic would not substantively contribute to its presence among environmental microbes; and (v) is the resistance to a clinically relevant antibiotic. These issues must be considered as part of a rigorous analysis that balances benefit with patient or consumer safety and public health concerns. The analysis must consider if the probiotic is being developed for broad distribution to generally healthy consumers (foods or supplements, for which a reasonable certainty of no harm is the general standard) or restricted distribution for patient populations (drugs). Since probiotics have the potential to exacerbate54 or mitigate55 the reservoir of AR genes harbored in humans, a case-by-case approach to safety is likely needed. Previous authors have suggested systematic approaches to considering this issue.12,, 14
Risk assessments for AR genotypes or phenotypes in probiotics are evolving, accommodating rapidly progressing science. The judgments on how to consider phenotype vs genotype or gene presence vs gene transferability can be expected to change based on research findings. Further, genetic modification methods may be useful to modify or eliminate the resistance elements of concern. Depending on the technology employed, this option may be suitable for probiotics for food applications (e.g., plasmid curing) or for next-generation probiotics that may be commercialized via the drug pathway.
We therefore are aligned with current recommendations that all probiotic strains should be screened for AR phenotypes and genotypes. The basis of any phenotypic resistance that is outside the norm for the species must be investigated further. If genes are harbored for antibiotics that are declared critically important by the World Health Organization,35, 36 the probiotic should be subjected to a rigorous risk/benefit analysis that balances patient and consumer safety as well as public health concerns. The potential of genetic transfer via transformation poses a low risk, which nevertheless warrants further study. Manufacturers of historical strains that may not have undergone this approach to AR risk assessment should reevaluate strains to assure compliance.
In the context of understanding the risk that probiotic-borne AR genes pose, it should be noted that this caution is not extended to most wild strains of food fermentation microbes, which are consumed live or dead in great numbers in the diets of many populations.56 Equally, fecal microbial transplants are not subjected to any AR gene-related restrictions despite the near certainty that many mobilizable AR genes are present in every fecal preparation. Lactic acid bacteria, which are used in the manufacture of fermented foods, have been shown to harbor AR genes.43 Horizontal transfer of AR genes from environmental microbes is also a possibility.57 The presence of AR genes in these microbes is largely unknown, yet there is no regulatory or scientific call to restrict the consumption of fermented foods on this basis.
Invasive infection
On rare occasions, probiotics may translocate from the gastrointestinal tract, resulting in invasive infection. A systematic review was conducted of sepsis, bacteremia, and fungemia associated with probiotic administration in children between 1995 and 2021. Of the 49 invasive infections reported, sepsis was most common. The majority of the children meeting the clinical definition of sepsis were under two years old and had a predisposing condition such as prematurity or an indwelling intravenous catheter, and 94% were treated successfully with antimicrobial therapy.58 The actual frequency of probiotic-related invasive infection is difficult to determine for multiple reasons. Most of the published case reports occurred outside the context of clinical trials, thus the number of patients receiving probiotics without adverse effects is unclear. Not all clinical laboratories routinely culture and identify probiotic organisms from blood cultures, and harms reporting in probiotic trials often is incomplete.30 Nonetheless, culture-proven invasive infection is studied as a primary or secondary outcome in most trials that administer probiotics to preterm neonates, and network meta-analyses suggest that probiotic administration does not increase rates of sepsis in this vulnerable population.59 However, the true frequency of probiotic-associated sepsis remains uncertain. We note that Hempel and colleagues5 concluded, “Across studies, there was no indication that critically ill and high-risk participants taking probiotics were more likely to experience AEs than control participants with the same health status.” We therefore conclude that invasive infections and sepsis should be monitored diligently in clinical settings and reported fully in all probiotic trials. Using strain-level molecular techniques, clinical isolates should be compared to the administered probiotic. A molecular match of supplemented probiotic microorganism(s) to invasive clinical isolate(s) supports an association between probiotic and systemic infection. Manufacturers should identify and publicize the antibiogram of each commercialized probiotic strain, providing an empirical course of treatment if needed. When assessing the safety of any next-generation probiotic, the potential for translocation should be determined and risks weighed against benefit.
Probiotic impact on drug function
The gut microbiota can have both direct and indirect effects on the metabolism of drugs, with consequences for both efficacy and toxicity.60, 61 For example, it has been known since the early seventies that the urinary excretion62 of total sulfanilamide in rats receiving the azo drugs Prontosil or Neoprontosil orally is reduced through the action of microbial azoreductase when the rats are treated with antibiotics,63 illustrating that the drug activation can be mediated by the microbiota. Other important microbiota-driven drug64–66 metabolisms have been described,65 such as decarboxylation (L-dopa67), sulfation (acetaminophen65) dehydroxylation (caffeic acid and L-dopa65), demethylation (methamphetamine65), dehalogenation,68 and acetylation/diacylation (salicylic acid to form aspirin65). Drug-related toxicity can also be reduced by the microbiota. A well-known example is glucuronidation,65 a conjugate hydrolysis reaction that links glucuronic acid to a substrate by an UDP-glucuronosyltransferase into hydrophilic and negatively charged glucuronides.69 Many anaerobic bacteria can induce β-glucuronidases, an enzyme able to deconjugate xenobiotics and endogenous compounds detoxified earlier via the glucuronidation pathway. This deconjugation can enhance enterohepatic recirculation of toxins, hormones, and various drugs as well as the formation of local carcinogens. Excess amounts of the β-glucuronidases may therefore increase the risk of colon cancer development. However, a certain amount of β-glucuronidase activity is important to guarantee enterohepatic recirculation of essential compounds such as vitamin D, thyroid hormone, or estrogen.
The ability of probiotics to impact drug function may have safety consequences. A relatively new discipline, toximicrobiomics or pharmacomicrobiomics, studies the interactions between the microbiota and xenobiotic compounds.70,, 71 Examples such as described by Dikeocha et al.71 indicate that it might be important to understand the interplay of microbiota diversity, diet and drug disposition and response and how this may impact future personalized medicine.72–74
Research is needed to identify drug-modifying enzymes and to confirm the relevance of these enzymes in vivo. The presence of such enzymes in a probiotic and evidence of in vitro functionality does not prove these activities would occur in a host. Further, evidence that such enzymes act to an extent that would hamper drug efficacy prior to the drug being absorbed is needed. The nascent nature of this research suggests that it is too early to make specific recommendations. Research focused on developing screens for the presence of enzymes that might metabolically affect specific drugs and databases indicating the related genomic sequences would move this field forward. The end goal would be to identify probiotics that encode enzymes of concern to be able to advise about probiotic – drug incompatibility.
Long-term risks
Long-term colonization
The efficacy of a probiotic microbe is not dependent on an ability to colonize the host long-term. Long-term colonization is normally taken to mean that an acutely administered microbe is still detectable from the host weeks or months after dosing has ceased. For an orally administered probiotic, this would mean that the microbe must be actively replicating and that it has established an ongoing presence within the host. Evidence accumulated to-date on recovery of probiotics from feces indicates that most current probiotics do not colonize.75,, 76 This almost certainly results from an inability to compete with the resident microbiota. In 1934, the famous Dutch botanist and microbiologist Baas Becking stated, “Everything is everywhere, but the environment selects.” cited in.77 In accordance with this concept, in most individuals simply encountering a microbe does not result in colonization, but if an ‘open niche’ is available, an externally applied microbe may be able to fill that niche and colonize.
Research interest has grown to understand what benefits could be provided by strains that are “native” or adapted to the host (as opposed to adapted to growth in large-scale production fermenters, food fermentations, or surviving while sitting on the shelves of traditional retail outlets). Indeed, efforts are underway to achieve better persistence of probiotic strains in human and other hosts using several methods. These efforts include manipulating host diet,78 adding prebiotics to support the strain’s growth,79,, 80 more precise targeting for adapted strains using computational techniques,81 and dose optimization.82 Such approaches have led to a new wave of strains that are showing longer term persistence in the host compared to previously published strains. While the array of benefits that are provided by these strains is outside of the scope of this review, this type of capability raises questions for scientists, consumers, and regulators on the long-term safety of these strains. There are now several examples, discussed below, of data in humans demonstrating longer-term engraftment in some study participants than previously shown possible with traditional strains, and this persistence was largely accomplished using autochthonous strains with provenance and physiological adaptations suggesting they are part of the normal human microbiota.
An early example of this phenomenon was the discovery that Bifidobacterium longum subp. longum AH1206 persisted for 6 months in some adults administered the strain.83 Likewise, Bifidobacterium adolescentis IVS-1 colonized for 4 weeks post-administration in some adults.80 A mixture of A.muciniphila, Clostridium beijerinckii, Clostridium butyricum, Bifidobacterium longum subsp. infantis and Anaerobutyricum hallii given to adults showed all strains persisted in some subjects to varying degrees for 4 weeks post administration.84 Evidence suggests B. longum subp. infantis EVC001 persisted in infants that were fed the strain for approximately one year after administration of the probiotic was discontinued.85, 86 Lactiplantibacillus plantarum ATCC 202,195 administered to infants appeared to persist for nearly 6 months based on analysis of plated isolates.87 Four out of a mixture of five probiotic strains in a commercial product were able to persist for several months post-supplementation when administered to premature infants.29
Persistent probiotics are also not limited solely to gastrointestinal niches and digestive health applications. Several vaginal strains including Lactobacillus crispatus CTV-05,88 L. rhamnosus GR-1, and Limosilactobacillus reuteri RC-14,89 all persist in the vaginal environment on the order of weeks to months. Administration of B. animalis subsp. lactis HN019 for 30 days resulted in its recovery for at least 60 days from the gingiva.90 Several commercial efforts are underway to identify and test colonization of the skin microbiota with probiotic strains adapted for that environment.91 These examples demonstrate the direction new probiotic strain discovery is taking. Strategies specifically aimed at developing long-term colonizing probiotics should take a risk-benefit approach.
What are the safety concerns? In most instances colonization with a probiotic derived from the common commensal microbiota at low levels should not be problematic to host health. Even high levels of a microbe with no obvious virulence potential should not negatively impact host health. However, it is conceivable that there could be risks associated with the increased exposure inherent to long-term colonizing probiotics. Potential risks include: (i) The probiotic could displace a microbe performing an important function; (ii) the probiotic could negatively impact the structure and function of the surrounding microbiota; and (iii) if the normal gut barrier is breached, a probiotic could access the systemic circulation, resulting in invasive infection. This last example was observed in a small but significant increase in Lactobacillus bacteremia in intensive care unit patients receiving probiotics in a Boston hospital, albeit this was not linked directly to colonization of the probiotic.92 Lack of probiotic colonization allows the prescribing physician or consumer to retain control of the dosing regimen, rather than depend on a self-replicating probiotic. Knowing the antibiotic sensitivity profile of a particular probiotic provides a strategy for eradication, if needed, and thus reduces the risk of long-term colonization in the unlikely case such risks should become apparent.
What are the potential benefits? One could make an argument that the long-term presence of the probiotic, which by its definition imparts a health benefit, could be an efficient and effective way of delivering long-term health benefits. Indeed, a microbe that can permanently occupy a vacant niche and provide a missing metabolic function – such as the ability to metabolize human milk oligosaccharides in an infant – that contributes to host health could represent an excellent probiotic candidate. Another consideration is that if the niche is destined to be occupied, would a long-term colonizing probiotic be a safer, more desirable occupier than an unknown microbe?
This new wave of strains that appear better adapted for establishing a presence and living in the human body has potential for achieving distinct and superior benefits. For example, imparting enzymatic capacity to compensate for a metabolic disorder such as phenylketonuria could be best achieved by a long-term colonizing probiotic. With our current understanding, we recommend that the development of a long-term colonizing probiotic be done only with a clear objective of achieving benefits not easily, reliably, or economically attainable otherwise and with weighing the risks against those clearly defined benefits. We recommend that careful consideration be dedicated to determining what long-term safety data might be relevant to probiotic strains that persist in the host. Research should be conducted to determine relevant acute exposure tests and biomarkers that are useful for assessing safety of long-term colonizing probiotics.
Assessment of microbiota composition or function alterations
Microbiome assessments conducted in conjunction with probiotic intervention trials have been undertaken primarily to address microbiota-mediated mechanisms driving efficacy, not as a measure of safety. But recent studies showing that a certain blend of live strains delayed microbiota composition recovery after antibiotic treatment highlights the potential role that microbiota readouts might have in addressing long-term safety. This study showed that humans and mice treated with antibiotics and then a probiotic mixture comprising 11 strains delayed for at least 6 months the recovery of gut mucosal and fecal microbiome composition and function to previous states compared to their counterparts, who did not receive probiotics.93 In fact, their gut microbiomes assumed a new steady state during probiotic administration and thereafter, which was significantly divergent from its baseline, although the possible benefit of this divergence was not considered. This study and others also demonstrated a lower number of observed species in the gut microbiota of probiotics-treated hosts after antibiotics.93–95 While these findings can be potentially harnessed to remodel the microbiota to a more desirable configuration, they might also raise a safety concern as microbiota alterations and reduced alpha diversity may be associated with infectious, inflammatory, and metabolic sequelae.96 Furthermore, a post-hoc analysis of the previous study revealed that probiotics administered after antibiotics caused an expansion in antibiotic resistance genes within the mucosal microbiota, in particular to vancomycin; however, this in-silico finding was not confirmed in-vivo.54 Vancomycin resistance manifested as an expansion of the vanG and vanSD genes (in humans and mice, respectively). The probiotic species were not the source for these genes, but came from the bloom of some members of the microbiota, specifically Clostridium, Blautia, and Romboutsia, which were carrying those genes. Taken together, there is evidence that the probiotic blend administered in this study given after antibiotic treatments alters the microbiota structure and function. However, there is no clear link between these alterations and clinical AEs nor is there evidence that this observation can be extended to other probiotic preparations. We therefore do not recommend that microbiota community composition and function analyses be routine for probiotic safety assessments, but recognize that profiling of microbiota structure and function may be useful for testing mechanisms and hypotheses. Research is needed to understand the clinical implications of any observed microbiota structure of function alterations.
What long-term safety studies are indicated?
Long-term safety studies for probiotics are much less well defined than those addressing acute safety issues. For drugs and biologics, subacute and chronic toxicities are important for revealing any detrimental effects associated with repeated dosing and/or associated bioaccumulation that may occur. In our context, bioaccumulation can refer to microbial metabolites but also microbial proliferation and colonization. These types of studies are usually conducted in animal models using validated procedures designed to provide insight into risk for humans. These studies are inherent to the drug development process prior to clinical use.97 Using the drug development pathway for FDA as a guide, this issue of long-term safety generally falls into a post-market monitoring system upon agreement with the manufacturer for submitting safety updates along with voluntary consumer and physician reporting of AEs to MedWatch.98 Long-term (estimated at >6 months) safety trials in humans may not be required for drug approval, although this would be assessed on a case-by-case basis. For example, most approved biologics for ulcerative colitis have been approved in recent years with little long-term data and some conflicting safety data. A literature review in this area revealed such studies have follow-ups that range from 16 to (at most) approximately 24 months stratified by collecting reports of AEs and serious AEs while monitoring and connecting to various other outcomes.99 Probiotics, which are often sold as supplements and not as drugs (live biotherapeutic products), lack the rigorous reporting requirements for post-market safety, although a formal process for reporting problems with dietary supplements exists. In the United States, MedWatch provides health professionals, patients, and consumers an avenue to report safety concerns for FDA-regulated product.98 Few clinical trials on probiotics follow study cohorts long-term, leaving a gap in long-term data.
Based on the traditional toxicological designs used to assess the safety of small molecules, long-term chronic studies in animal models are defined typically as 6 months to 1 year in duration with a priori experiential knowledge or predisposition toward affected organs, reversibility of toxicity, (no) observed effect levels, and quantifying clinical risk at expected dose for long-term treatment.100, 101 Translating this preclinical framework to next-generation live biotherapeutics, which lack history of safe use, would suggest that 12-month studies in humans would be in line with expectations for new molecular agents/targets when little-to-no post-market experience is available and should potentially be conducted concurrently with Phase III clinical trials. However, most of the established toxicological frameworks have been developed over the years from pharmacokinetic/toxicokinetics and a variety of preclinical testing modalities. However, their relevance to probiotics that do not generally enter the bloodstream or exhibit traditional small-molecule decay rates is not clearly apparent.
Given this backdrop, the safety challenge is defining relevant outcomes and pathways that may span the cadre of intended organisms and their metabolic potentials within a microbial community setting of the gut.102 This becomes a confounding proposition given our current limited understanding of the microbiota, colonization, functional parameters, and their contribution to health and well-being. Predictive biomarkers of health and disease based on microbiota community structure and function are needed.102 Some relevant features might include thresholds affecting diversity indices and/or microbial community alterations that indicate concern for reflexivity to normalcy or community structures indicative of, for example, pro-inflammatory, pro-obesity, disrupted immune homeostasis, metabolic syndrome/diabetes dispositions or other additional conditions that may require basic research to resolve validated metagenomic markers.102,103 Published time-course studies and follow-up in study subjects would provide some basis for establishing the validity and usefulness of such parameters and whether they are generalizable across populations, thereby contributing to the development and continuing innovation of live biotherapeutic products. However, regulatory requirement prior to approval process in this regard may overreach without rational basis or may simply be unachievable with currently available tools.
It is clear that the state of science and understanding of key metrics for safety in specialized population or disease states (and associated model systems) have not yet achieved a literature base or consensus for establishing long-term safety recommendations for probiotics. Adding to this uncertainly is the potential for rationally designed microbial consortia with functional properties that are synergistic. As we learn more about the ways in which the microbiota impacts human physiology in the long-term, specific approaches to long-term follow-up may become warranted.
Based on current FDA requirements for biologic drugs, including fecal microbial transplants, we do not recommend specific tests or length of follow-up to address long-term issues. Research in humans focused on determining if a probiotic changes the long-term microbiota composition or function should include collecting data on AEs, similar to acute studies. We advise research into animal models, especially as applied to next-generation strains, to further our mechanistic understanding of how to measure potential long-term effects. In accordance with regulatory requirements for foods, supplements or drugs, companies must track and report adverse events.
Vulnerable target populations
Long-term studies designed to demonstrate probiotic safety in populations at risk (such as individuals with weakened/impaired immune function, aged people, newborns, particularly preterm infants) are scarce. Although beneficial effects of probiotics in such groups are reported, immunocompromised hosts might be at higher risk for AEs due to their reduced ability to defend against a microbial challenge.104 Tracking the long-term impact for weeks or months of probiotics administered through infant formula from birth is warranted. The homogeneous diet and developing gut microbiota may enable such exposure to probiotics to permanently impact the development of their microbial ecosystem.
Evidence from short-term observations suggest that certain probiotic strains might behave as opportunistic pathogens in populations who are immunocompromised, stressed, aged, or newly born.105 AEs include life-threatening pneumonia,106 endocarditis,107–111 and sepsis.112–115 In general, it has been suggested that in vulnerable populations, the presence of a single major risk factor, such as immunocompromised state, or more than one minor risk factor, merits caution in using probiotics.116 However, to the extent compelling evidence exists that probiotics can benefit some vulnerable populations, their use should be considered. Based on the available data, extra monitoring is warranted when probiotics are administered to vulnerable target populations.
Risks of probiotics to pregnant and lactating women have been reviewed.117–119 Of 100 eligible studies of probiotic administration during pregnancy, only 28 reported AEs. Of these, only 11 reported AEs that potentially could have a causal relationship with the treatment, including gastrointestinal problems, nausea, and headache; but no serious health concerns were reported for mother or infant.117 The remaining 72 studies did not report AEs. One study reported an increased risk of vaginal discharge and changes in stool consistency when administering L. rhamnosus GR-1 and L. reuteri RC-14,118–120 but a recent study did not confirm this observation.121 The reviewed publications suggest that probiotics do not appear to pose a safety concern for this population. However, the findings of a Cochrane review122 showed an increased risk of pre-eclampsia when data from four trials in obese women – who are at an increased risk for pre-eclampsia – were pooled (31 cases of pre-eclampsia in 472 women who took probiotics versus 17 in 483 women in the placebo groups). Although the data are not robust, we still recommend that probiotics for mild to morbidly obese pregnant women be administered only with concomitant monitoring for potential risk of pre-eclampsia.
Some concerns have been expressed regarding metabolic activity of some probiotics used in certain populations. D-lactic acidosis can occur in people with surgically altered gut anatomy, such as short bowel or bariatric surgery, resulting from activity of resident microbes.123 There are few published accounts linking this to probiotic supplementation.124 Lack of data in preterm infants led to a caution about using D-lactate-producing probiotics in this population,125 although a controlled trial in healthy full-term infants showed that D-lactate-producing L. reuteri DSM-17938 did not result in acidosis126 and probiotic-containing infant formulas were not associated with acidosis.127
Preterm infants warrant special consideration given the unique window of opportunity for modulation of microbiota structure and function amidst numerous competing prenatal, perinatal, and postnatal factors.128 Neonatal microbiota-targeting therapies have the potential to influence host biology throughout the lifespan, either by introducing allochthonous microbial strains when conditions may be most permissive to colonization, or by influencing early developmental trajectories of vital organs including the brain.129 To date, there is little evidence to suggest that early-life probiotic supplementation adversely influences neurodevelopmental outcomes. In one follow-up study of 1,099 very preterm infants, there was no difference in major neurodevelopment outcomes at 3–5 years of age in surviving infants who had received compared to those who had not received probiotics. Intriguingly, deafness was less prevalent in the probiotic-treated children, and this could not be attributed to differences in the numbers of courses of antibiotics or to the total days of vancomycin or gentamicin received.130
Other studies have identified a link between early-life microbiota alterations and obesity. A large cohort study of 333,353 children in the United States reported that prescriptions of antibiotics and acid-suppressive medications in the first two years of life are associated with obesity later in childhood; these associations were strengthened with each additional class of these microbiota-altering drugs and with each additional 30-day prescription.131 Similarly, exposure to household disinfectants early in life is associated with higher body mass index at three years of age in a cohort of 757 Canadian infants.132 Importantly, studies evaluating rates of childhood obesity following probiotic therapy in the perinatal and infant period have not reported any AEs with respect to body mass index.133, ,134 Thus, the limited evidence available does not suggest that early-life probiotic use increases the risk of adverse outcomes in childhood. There are not enough data to determine whether potential associations may exist between perinatal probiotic use and AEs in adulthood. Therefore, we encourage a minimum of 2-year (when most outcomes are no longer corrected for prematurity) follow up from studies of premature infants who received probiotics or not in the perinatal period to compare metabolic, allergic, immune, and other health outcomes.
Probiotic quality considerations
In general, high quality, safe probiotic products are produced under dietary supplement and food regulations, although there have been incidences of noncompliance documented for supplements15 and calls for improved product quality.135 Dietary supplements are a category of products developed to supplement the diet of the generally healthy population, not to treat or prevent disease. This is important because while the safety standard for dietary supplements requires that they will reasonably expected to be safe under the labeled conditions of use, they do not need to be established as safe for more vulnerable patient populations.9 The intended use is crucial in establishing essential efficacy and safety requirements. In different jurisdictions, various guidelines and regulations exist for good manufacturing practices. In the United States, the Food and Drug Administration distinguishes, among others, current good manufacturing practices for food and drugs:
For food and dietary supplements: These describe the “methods, equipment, facilities, and controls for producing processed food”. These are meant to ensure that the food is safe to eat.136
For drugs: These assure “the identity, strength, quality, and purity of drug products by requiring that manufacturers of medications adequately control manufacturing operations”.137
The onus is on manufacturers to establish appropriate product specifications based on intended use and acceptable risk levels. Reputable manufacturers establish rigorous purity, potency, and identity quality standards consistent with the intended population and sufficient for that use. These standards require quality control and quality assurance protocols.
Quality specifications sufficient for vulnerable populations can be developed.138 Testing requirements more stringent than those sufficient for healthy populations can be requested from product manufacturers (Table 1). Adherence to these standards can be verified independently by a third party with this expertise.135 Even if adherence to these higher standards is not referenced on product labels, certificates of analysis should specify testing results. Hospital pharmacists who stock formularies and scientists who aim to investigate probiotics in these vulnerable (patient) populations should work with the supplier to provide extra product testing and visibility of those results. Pharmacies can negotiate quality agreements with vendors that would delineate their expectations for the strains present, their potency, the limits for contaminants (microbiological and others), and other criteria. This agreement could also mandate that any product change – as defined in the agreement – would require the vendor to notify the pharmacist or researcher. Such an agreement would increase the burden on a hospital pharmacist or an academic investigator but would establish the testing standards a pharmacy would expect of the products it stocks. A sophisticated dietary supplement provider should be able to assure the hospital formulary and investigator of their product quality and the process they have followed to assure and control this.
We recommend that probiotic dietary supplements targeted for vulnerable populations undergo third-party verification of product quality (purity, potency, and identity), which are accurately communicated on product labels. Further, such products should undergo testing to meet quality standards appropriate for that population. Direct agreements with probiotic manufacturers may be useful for developing products to meet stricter quality control standards. We also recommend that approaches to product labeling be reviewed to enable stricter standards to be communicated on product labels.
Toward progress in reporting adverse events
Reporting AEs in randomized controlled trials is critically important to understanding the trade-offs between the benefits and harms of interventions. Missing or insufficient reporting of harm-related data is not specific to the probiotic field. Similar issues have been reported regarding other interventions.139–142 Still, clinical researchers should follow long-standing recommendations for reporting harms as delineated in the Consolidated Standards of Reporting Trials Group published guidelines for complete and detailed reporting of harms, known as the CONSORT Extension for Harms.143 Authors should provide a balanced discussion of the benefits and harms. We recommend that in all clinical trials on probiotics, data on adverse events should be collected. Events should be listed and defined, with reference to standardized criteria where appropriate. For each study arm, the absolute risk of each adverse event, using appropriate metrics for recurrent events, and the number of participants withdrawn due to harms should be presented. We also recommend that the authors should provide a balanced discussion of the benefits and harms.
Conclusions
We met to identify emerging acute and long-term risks associated with probiotics and to update recommendations pertaining to probiotic safety. Probiotic safety encompasses properties inherent to the probiotic, to the consumer/patient, and to the manufacturing process (contaminated probiotics products represent a safety concern). Table 2 summarizes our recommendations. Some should be implemented promptly but others warrant further study before actionable recommendations can be developed.
Table 2.
Summary of key recommendations regarding acute and long-term safety concerns for probiotics. AR, antibiotic resistance.
| Safety risks | Recommendation |
|---|---|
| ACUTE SAFETY | |
| Microbiome assessments |
|
| Antibiotic resistance |
|
| Invasive infection |
|
| Probiotic:drug compatibility |
|
| Vulnerable populations |
|
| LONG TERM SAFETY | |
| Long-term studies |
|
| Long-term colonization |
|
| Microbiome assessments |
|
| Vulnerable populations |
|
| GENERAL RECOMMENDATIONS | |
| Probiotic quality |
|
| Adverse event reporting |
|
| Probiotic product labeling |
|
Potential long-term concerns are difficult to address as data are limited. But for probiotics, an approach should align with regulatory approaches used for other biologics. Ongoing research will surely bring to light new long-term safety implications that will need to be considered in safety assessments. But applying biomarkers and other outcomes that are often used in short-term assessments may not be appropriate to address long-term safety implications. For example, if a strain colonizes the host, enhanced long-term evaluations are recommended. However, assessment of microbiome alterations is likely insufficient for this task, since the clinical implications of these alterations are unclear. Research is needed to clarify which types of high-risk groups require closer long-term follow-up. For example, mild to morbidly obese women may need closer monitoring during pregnancy and additional long-term follow-up studies are warranted for premature infants.
No assurance of absence of harm can be guaranteed with any food, supplement, or medical intervention. This paper aims to engender those tasked with assessing safety of probiotics to consider the emerging issues described herein. In some cases, regulatory frameworks notwithstanding, the risk-to-benefit ratio must be considered. We hope this paper helps guide the scientific, regulatory, and medical communities about considerations for this judgment.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Funding Statement
ISAPP provided travel funding for non-industry-affiliated members of this expert panel. ISAPP is funded by equal contributions from member companies involved in the development, assessment, or marketing of probiotic or prebiotic products. Although ISAPP receives funds to support its activities from commercial sources, its activities and positions are set by an all-academic, volunteer board of scientists. Industry members have no control over the positions of the organization.
Disclosure statement
DM has been a paid consultant for HowarU and Bayer, has done legal work for VSL#3, and is President of the ISAPP board, a non-paid position. CH has consulted for Kyowa Hakko, Adiso Therapeutics and Winclove BV and serves as a non-paid member of the Board of Directors of ISAPP. GAP declares no conflicts of interest. ALS declares no conflicts of interest. NZ declares no conflicts of interest. MCC has been paid to speak at meetings by HIPP, Danone, Nutricia, Nestlé Nutrition Institute and Mead Johnson. LM has been a paid consultant for Sanofi, Bayer, Danone, and Hamni. CAE declares no conflicts of interest. Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention. C.A. Elkins contributions are primarily attributed to the section titled “ What long-term safety studies are indicated?” and conceptual elements within the section titled “AR Horizontal transfer of antibiotic resistance genes.” GAM is employed by the Société des Produits Nestlé S.A. ZTL is an employee of Synbiotic Health, Inc. BP is employed by Yakult Europe BV and serves in non-remunerated positions as president of the Pharmabiotics Research Institute, board member of Lactic Acid Bacteria Industrial Platform and board chair of International Life Sciences Institute - EU. GL is employed by Chr. Hansen. MIP is employed by Winclove Probiotics and serves as a consultant for academic and industrial representatives in the field of microbiome and probiotics. Her consultancy clients had no role in drafting this manuscript or the decision to submit the work for publication. ACO is employed by International Flavors & Fragrances. HS has participated as a clinical investigator, and/or advisory board member, and/or consultant, and/or speaker for: Arla, BioGaia, Biocodex, Danone, Dicofarm, Nestlé, NNI Nutricia, Mead Johnson and serves as a non-paid member of the Board of Directors of ISAPP. DJT has been a paid consultant for Synbiotic Health, Inc., and International Fragrance & Flavors and serves as a non-paid member of the Board of Directors of ISAPP. MES has been a paid consultant for Bayer, Bill and Melinda Gates Foundation, California Dairy Research Foundation, Danone North America, Pepsico, Smith, Gambrell & Russell, LLP, and Winclove, has received honoraria for speaking from Associated British Foods, Biocodex, European Federation of Associations of Dietitians, fairlife, Allergosan, Probi, and Sanofi, is paid as the executive science officer of International Scientific Association for Probiotics and Prebiotics, and has served on advisory boards for Cargill, Danone North America, Danone, Yakult, and Winclove.
Abbreviations
- AE
adverse event
- AR
antibiotic resistance
- GMP
good manufacturing requirements
- MIC
minimum inhibitory concentration
- FDA
U.S. Food and Drug Administration
References
- 1.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–22. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 2.Mogensen G, Salminen S, O’brien J, Ouwehand AC, Holzapfel W, Shortt C, et al. Food microorganisms - health benefits, safety evaluation and strains with documented history of use in foods. Bulletin of the IDF. 2002;377:4–9. [Google Scholar]
- 3.Goldenberg JZ, Yap C, Lytvyn L, Lo CK, Beardsley J, Mertz D, Johnston BC.. Probiotics for the prevention of clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095. doi: 10.1002/14651858.CD006095.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Q, Goldenberg JZ, Humphrey C, El Dib R, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4:CD004827. doi: 10.1002/14651858.CD004827.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hempel S, Newberry S, Ruelaz A, Wang Z, Miles JN, Suttorp MJ, Johnsen B, Shanman R, Slusser W, Fu N, et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technol Assess (Full Rep). 2011;200:1–645. [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders ME, Merenstein DJ, Ouwehand AC, Reid G, Salminen S, Cabana MD, Paraskevakos G, Leyer G. Probiotic use in at-risk populations. J Am Pharm Assoc. 2003. 2016;56(6):680–686. doi: 10.1016/j.japh.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 7.van den Nieuwboer M, Claassen E. Dealing with the remaining controversies of probiotic safety. Benef Microbes. 2019;10(6):605–616. doi: 10.3920/BM2018.0159. [DOI] [PubMed] [Google Scholar]
- 8.Hill C. Balancing the risks and rewards of live biotherapeutics. Nat Rev Gastroenterol Hepatol. 2020;17(3):133–134. doi: 10.1038/s41575-019-0254-3. [DOI] [PubMed] [Google Scholar]
- 9.Rouanet A, Bolca S, Bru A, Claes I, Cvejic H, Girgis H, Harper A, Lavergne SN, Mathys S, Pane M, et al. Live biotherapeutic products, a road map for safety assessment. Front Med (Lausanne). 2020;7:237. doi: 10.3389/fmed.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuntar I, Petric Z, Bursac Kovacevic D, Putnik P. Safety of probiotics: functional fruit beverages and nutraceuticals. Foods. 2020;9(7):947. doi: 10.3390/foods9070947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koutsoumanis K, Allende A, Alvarez‐ordóñez A, Bolton D, Bover-cid S, Chemaly M, Davies R, De Cesare A, Hilbert F, Lindqvist R, et al. Update of the list of QPS-recommended microbiological agents intentionally added to food or feed as notified to EFSA 16: suitability of taxonomic units notified to EFSA until march 2022. EFSAJ. (2022), 20(7). 2022. doi: 10.2903/j.efsa.2022.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pariza MW, Gillies KO, Kraak-Ripple SF, Leyer G, Smith AB. Determining the safety of microbial cultures for consumption by humans and animals. Regul Toxicol Pharmacol. 2015;73(1):164–171. doi: 10.1016/j.yrtph.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Holzapfel W, Arini A, Aeschbacher M, Coppolecchia R, Pot B. Enterococcus faecium SF68 as a model for efficacy and safety evaluation of pharmaceutical probiotics. Benef Microbes. 2018;9(3):375–388. doi: 10.3920/BM2017.0148. [DOI] [PubMed] [Google Scholar]
- 14.Roe AL, Boyte ME, Elkins CA, Goldman VS, Heimbach J, Madden E, Oketch-Rabah H, Sanders ME, Sirois J, Smith A. Considerations for determining safety of probiotics: a USP perspective. Regul Toxicol Pharmacol. 2022;136:105266. doi: 10.1016/j.yrtph.2022.105266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen PA. Probiotic safety—no guarantees. JAMA Intern Med. 2018;178(12):1577–1578. doi: 10.1001/jamainternmed.2018.5403. [DOI] [PubMed] [Google Scholar]
- 16.Freedman SB, Schnadower D, Tarr PI. The probiotic conundrum: regulatory confusion, conflicting ctudies, and safety concerns. JAMA. 2020;323(9):823–824. doi: 10.1001/jama.2019.22268. [DOI] [PubMed] [Google Scholar]
- 17.Poindexter B, Committee on F, Hand, Newborn I, Adams-Chapman I, Aucott SW, Puopolo KM, Goldsmith JP, Kaufman D, Martin C, et al. Use of probiotics in preterm infants. Pediatrics. 2021; 147(6). doi: 10.1542/peds.2021-051485. [DOI] [PubMed] [Google Scholar]
- 18.Cassone M, Serra P, Mondello F, Girolamo A, Scafetti S, Pistella E, Venditti M. Outbreak of Saccharomyces cerevisiae subtype boulardii fungemia in patients neighboring those treated with a probiotic preparation of the organism. J Clin Microbiol. 2003;41(11):5340–5343. doi: 10.1128/JCM.41.11.5340-5343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennequin C, Kauffmann-Lacroix C, Jobert A, Viard JP, Ricour C, Jacquemin JL, Berche P. Possible role of catheters in Saccharomyces boulardii fungemia. Eur J Clin Microbiol Infect Dis. 2000;19(1):16–20. doi: 10.1007/s100960050003. [DOI] [PubMed] [Google Scholar]
- 20.Bongaerts GPA, Severijnen RSVM. A reassessment of the PROPATRIA study and its implications for probiotic therapy. Nat Biotechnol. 2016;34(1):55–63. doi: 10.1038/nbt.3436. [DOI] [PubMed] [Google Scholar]
- 21.Cabana MD, Salminen S, Sanders ME. Probiotic safety—reasonable certainty of no harm. JAMA Intern Med. 2019;179(2):276. doi: 10.1001/jamainternmed.2018.7498. [DOI] [PubMed] [Google Scholar]
- 22.Su GL, Ko CW, Bercik P, Falck-Ytter Y, Sultan S, Weizman AV, Morgan RL. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020;159(2):697–705. doi: 10.1053/j.gastro.2020.05.059. [DOI] [PubMed] [Google Scholar]
- 23.Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25(5):716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 24.Suez J, Zmora N, Elinav E. Probiotics in the next-generation sequencing era. Gut Microbes. 2020;11(1):77–93. doi: 10.1080/19490976.2019.1586039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wm DV, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–1032. doi: 10.1136/gutjnl-2021-326789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C, Derrien M, Levenez F, Brazeilles R, Ballal SA, Kim J, Degivry M-C, Quéré G, Garault P, van Hylckama Vlieg JET, et al. Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. Isme J. 2016;10(9):2235–2245. doi: 10.1038/ismej.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray KJ, Santee C, McCauley K, Panzer AR, Lynch SV. Gut Bifidobacteria enrichment following oral Lactobacillus-supplementation is associated with clinical improvements in children with cystic fibrosis. BMC Pulm Med. 2022;22(1):287. doi: 10.1186/s12890-022-02078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang CM, Tsai MH, Liao WC, Yang PH, Li SW, Chu SM, Huang H-R, Chiang M-C, Hsu J-F. Effects of probiotics on gut microbiomes of extremely preterm infants in the neonatal intensive care unit: a prospective cohort study. Nutrients. 2022;14(15):3239. doi: 10.3390/nu14153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samara J, Moossavi S, Alshaikh B, Ortega VA, Pettersen VK, Ferdous T, Hoops SL, Soraisham A, Vayalumkal J, Dersch-Mills D, et al. Supplementation with a probiotic mixture accelerates gut microbiome maturation and reduces intestinal inflammation in extremely preterm infants. Cell Host Microbe. 2022;30(5):696–711 e5. doi: 10.1016/j.chom.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Bafeta A, Koh M, Riveros C, Ravaud P. Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review. Ann Intern Med. 2018;169(4):240–247. doi: 10.7326/M18-0343. [DOI] [PubMed] [Google Scholar]
- 31.Food and Agricultural Organization of the United Nations and World Health Organization . Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. 2001. https://www.fao.org/3/a0512e/a0512e.pdf.
- 32.Food and Agricultural Organization of the United Nations and World Health Organization . Joint FAO/WHO working group report on drafting guidelines for the evaluation of probiotics in food. 2002. https://www.fao.org/3/a0512e/a0512e.pdf.
- 33.Morelli L, Sarra PG, Bottazzi V. In vivo transfer of pAmβ1 from Lactobacillus reuteri to Enterococcus faecalis. J Appl Bacteriol. 1988;65(5):371–375. doi: 10.1111/j.1365-2672.1988.tb01905.x. [DOI] [PubMed] [Google Scholar]
- 34.Crits-Christoph A, Hallowell HA, Koutouvalis K, Suez J. Good microbes, bad genes? the dissemination of antimicrobial resistance in the human microbiome. Gut Microbes. 2022;14(1):2055944. doi: 10.1080/19490976.2022.2055944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance . Critically important antimicrobials for human medicine. 2018. https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf.
- 36.World Health Organization . The WHO essential medicines list antibiotic book: improving antibiotic AWaReness. 2022. https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf.
- 37.European Food Safety Authority . Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012;10(6):2012. doi: 10.2903/j.efsa.2012.2740. [DOI] [Google Scholar]
- 38.Kowalska-Krochmal B, Dudek-Wicher R. The minimum inhibitory concentration of antibiotics: methods, interpretation, clinical relevance. Pathogens. 2021;10(2):165. doi: 10.3390/pathogens10020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Technical committee: iSO/TC 34/SC 5. Milk and milk products — determination of the minimal inhibitory concentration (MIC) of antibiotics applicable to bifidobacteria and non-enterococcal lactic acid bacteria (LAB). USI. 10932.2010. [Google Scholar]
- 40.Impey RE, Hawkins DA, Sutton JM, Soares da Costa TP. Overcoming intrinsic and acquired resistance mechanisms associated with the cell wall of gram-negative bacteria. Antibiotics (Basel). 2020;9(9). doi: 10.3390/antibiotics9090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elkins CA, Mullis LB. Bile-mediated aminoglycoside sensitivity in Lactobacillus species likely results from increased membrane permeability attributable to cholic acid. Appl Environ Microbiol. 2004;70(12):7200–7209. doi: 10.1128/AEM.70.12.7200-7209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stogios PJ, Savchenko A. Molecular mechanisms of vancomycin resistance. Protein Sci. 2020;29(3):654–669. doi: 10.1002/pro.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stefanska I, Kwiecien E, Jozwiak-Piasecka K, Garbowska M, Binek M, Rzewuska M. Antimicrobial susceptibility of lactic acid bacteria strains of potential use as feed additives - the basic safety and usefulness criterion. Front Vet Sci. 2021;8:687071. doi: 10.3389/fvets.2021.687071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morelli L, Matto J, Wilcks A, Aarts HJM, Bolton D. ACE-ART EU project for the evaluation of antibiotic resistance transferability in the food chain. NUTRAfoods. 2005;4:89–93. [Google Scholar]
- 45.U.S. Food & Drug Administration . GRN No. 49. Bifidobacterium lactis strain Bb12 and streptococcus thermophilus strain Th4. 2005. https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=49&sort=GRN_No&order=DESC&startrow=1&type=basic&search=bifidobacterium
- 46.McInnes RS, McCallum GE, Lamberte LE, van Schaik W. Horizontal transfer of antibiotic resistance genes in the human gut microbiome. Curr Opin Microbiol. 2020;53:35–43. doi: 10.1016/j.mib.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Winter M, Buckling A, Harms K, Johnsen PJ, Vos M. Antimicrobial resistance acquisition via natural transformation: context is everything. Curr Opin Microbiol. 2021;64:133–138. doi: 10.1016/j.mib.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Cj VW, Penders J, Jm VN, Nd M, Majumder S, Lb VA, Savelkoul PHM, Wolffs PFG. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front Microbiol. 2016;7:173. doi: 10.3389/fmicb.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, Sanders ME, Shamir R, Swann JR, Szajewska H, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18(9):649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.European Food Safety Authority . Scientific opinion on the safety of pasteurised akkermansia muciniphila as a novel food pursuant to regulation (EU) 2015/2283. EFSA J. 2021;19(9). doi: 10.2903/j.efsa.2021.6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.EFSA Panel on Nutrition NFaFAN . Safety of pasteurisedAkkermansia muciniphilaas a novelfood pursuant to Regulation (EU) 2015/2283. EFSA J. 2021;19:6780. [Google Scholar]
- 52.Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, Huynh W, Nguyen ALV, Cheng AA, Liu S, et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48:D517–25. doi: 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Institutes of Health . National Database of Antibiotic Resistant Organisms (NDARO). National Library of Medicine; 2022. https://www.ncbi.nlm.nih.gov/pathogens/antimicrobial-resistance/ [Google Scholar]
- 54.Montassier E, Valdes-Mas R, Batard E, Zmora N, Dori-Bachash M, Suez J, Elinav E. Probiotics impact the antibiotic resistance gene reservoir along the human GI tract in a person-specific and antibiotic-dependent manner. Nat Microbiol. 2021;6:1043–1054. doi: 10.1038/s41564-021-00920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guitor AK, Yousuf EI, Raphenya AR, Hutton EK, Morrison KM, McArthur AG, Wright GD, Stearns JC. Capturing the antibiotic resistome of preterm infants reveals new benefits of probiotic supplementation. Microbiome. 2022;10(1):136. doi: 10.1186/s40168-022-01327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell-Platt G. Fermented foods — a world perspective. Int Food Res J. 1994;27(3):253–257. doi: 10.1016/0963-9969(94)90093-0. [DOI] [Google Scholar]
- 57.Maeusli M, Lee B, Miller S, Reyna Z, Lu P, Yan J, et al.Horizontal gene transfer of antibiotic resistance from acinetobacter baylyi to escherichia coli on lettuce and subsequent antibiotic resistance transmission to the gut microbiome mSphere 2020; 5. 10.1128/mSphere.00329-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’agostin M, Squillaci D, Lazzerini M, Barbi E, Wijers L, Da Lozzo P. Invasive infections associated with the use of probiotics in children: a systematic review. Children (Basel). 2021;8(10):924. doi: 10.3390/children8100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morgan RL, Preidis GA, Kashyap PC, Weizman AV, Sadeghirad B, McMaster Probiotic P, Florez ID, Foroutan F, Shahid S, Zeraatkar D. Probiotics reduce mortality and morbidity in preterm, low-birth-weight infants: a systematic review and network meta-analysis of randomized trials. Gastroenterology. 2020;159(2):467–480. doi: 10.1053/j.gastro.2020.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson ID, Nicholson JK. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. 2017;179:204–222. doi: 10.1016/j.trsl.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dashnyam P, Mudududdla R, Hsieh TJ, Lin TC, Lin HY, Chen PY, Hsu C-Y, Lin C-H. β-Glucuronidases of opportunistic bacteria are the major contributors to xenobiotic-induced toxicity in the gut. Sci Rep. 2018;8(1):16372. doi: 10.1038/s41598-018-34678-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carmody RN, Turnbaugh PJ. Host-microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J Clin Invest. 2014;124(10):4173–4181. doi: 10.1172/JCI72335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gingell R, Bridges JW. Intestinal azo reduction and glucuronide conjugation of prontosil. Biochem J. 1971;125(2):24. doi: 10.1042/bj1250024Pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chung KT, Stevens SE Jr., Cerniglia CE. The reduction of azo dyes by the intestinal microflora. Crit Rev Microbiol. 1992;18(3):175–190. doi: 10.3109/10408419209114557. [DOI] [PubMed] [Google Scholar]
- 65.Klaassen CD, Cui JY. Review: mechanisms of how the intestinal microbiota alters the effects of drugs and bile acids. Drug Metab Dispos. 2015;43(10):1505–1521. doi: 10.1124/dmd.115.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pant A, Maiti TK, Mahajan D, Das B. Human gut microbiota and drug metabolism. Microb Ecol. 2022. doi: 10.1007/s00248-022-02081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP. Discovery and inhibition of an interspecies gut bacterial pathway for levodopa metabolism. Science. 2019; 364(6445). doi: 10.1126/science.aau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atashgahi S, Shetty SA, Smidt H, de Vos WM. Flux, impact, and fate of halogenated xenobiotic compounds in the gut. Front Physiol. 2018;9:888. doi: 10.3389/fphys.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang G, Ge S, Singh R, Basu S, Shatzer K, Zen M, Liu J, Tu Y, Zhang C, Wei J, et al. Glucuronidation: driving factors and their impact on glucuronide disposition. Drug Metab Rev. 2017;49(2):105–138. doi: 10.1080/03602532.2017.1293682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdelsalam NA, Ramadan AT, ElRakaiby MT, Aziz RK. Toxicomicrobiomics: the human microbiome vs. pharmaceutical, dietary, and environmental xenobiotics. Front Pharmacol. 2020;11:390. doi: 10.3389/fphar.2020.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dikeocha IJ, Al-Kabsi AM, Miftahussurur M, Ma A. Pharmacomicrobiomics: influence of gut microbiota on drug and xenobiotic metabolism. Faseb J. 2022;36(6):e22350. doi: 10.1096/fj.202101986R. [DOI] [PubMed] [Google Scholar]
- 72.Candeliere F, Raimondi S, Ranieri R, Musmeci E, Zambon A, Amaretti A, Rossi M.β-Glucuronidase pattern predicted from gut metagenomes indicates potentially diversified pharmacomicrobiomics.Front Microbiol.2022.13. 10.3389/fmicb.2022.826994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doestzada M, Vila AV, Zhernakova A, Koonen DPY, Weersma RK, Touw DJ, Kuipers F, Wijmenga C, Fu J. Pharmacomicrobiomics: a novel route towards personalized medicine? Protein & Cell. 2018;9(5):432–445. doi: 10.1007/s13238-018-0547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hassan M, Awan FM, Naz A, DeAndres-Galiana EJ, Alvarez O, Cernea A, Fernández-Brillet L, Fernández-Martínez JL, Kloczkowski A. Innovations in genomics and big data analytics for personalized medicine and health care: a review. Int J Mol Sci. 2022;23(9):4645. doi: 10.3390/ijms23094645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanders ME, Heimbach JT, Pot B, Tancredi DJ, Lenoir-Wijnkoop I, Lahteenmaki-Uutela A, Gueimonde M, Bañares S. Health claims substantiation for probiotic and prebiotic products. Gut Microbes. 2011;2(3):127–133. doi: 10.4161/gmic.2.3.16174. [DOI] [PubMed] [Google Scholar]
- 76.Khalesi S, Bellissimo N, Vandelanotte C, Williams S, Stanley D, Irwin C. A review of probiotic supplementation in healthy adults: helpful or hype? Eur J Clin Nutr. 2019;73(1):24–37. doi: 10.1038/s41430-018-0135-9. [DOI] [PubMed] [Google Scholar]
- 77.van Schaik W. Baas becking meets one health. Nat Microbiol. 2022;7(4):482–483. doi: 10.1038/s41564-022-01100-4. [DOI] [PubMed] [Google Scholar]
- 78.Tachon S, Lee B, Marco ML. Diet alters probiotic Lactobacillus persistence and function in the intestine. Environ Microbiol. 2014;16(9):2915–2926. doi: 10.1111/1462-2920.12297. [DOI] [PubMed] [Google Scholar]
- 79.Rattanaprasert M, Roos S, Hutkins RW, Walter J. Quantitative evaluation of synbiotic strategies to improve persistence and metabolic activity of Lactobacillus reuteri DSM 17938 in the human gastrointestinal tract. J Funct Foods. 2014;10:85–94. doi: 10.1016/j.jff.2014.05.017. [DOI] [Google Scholar]
- 80.Krumbeck JA. Characterization of the role of host and dietary factors in the establishment of bacteria in the gastrointestinal tract. Diss Theses Biol Sci. 2016;87. [Google Scholar]
- 81.Aggarwala V, Mogno I, Li Z, Yang C, Britton GJ, Chen-Liaw A, Mitcham J, Bongers G, Gevers D, Clemente JC, et al. Precise quantification of bacterial strains after fecal microbiota transplantation delineates long-term engraftment and explains outcomes. Nat Microbiol. 2021;6:1309–1318. doi: 10.1038/s41564-021-00966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taverniti V, Koirala R, Dalla via A, Gargari G, Leonardis E, Arioli S, Guglielmetti S. Effect of cell concentration on the persistence in the human intestine of four probiotic strains administered through a multispecies formulation. Nutrients. 2019;11(2):11. doi: 10.3390/nu11020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maldonado-Gomez MX, Martinez I, Bottacini F, O’callaghan A, Ventura M, van Sinderen D, Hillmann B, Vangay P, Knights D, Hutkins R, et al. Stable engraftment of bifidobacterium longum ah1206 in the human gut depends on individualized features of the resident microbiome. Cell Host Microbe. 2016;20(4):515–526. doi: 10.1016/j.chom.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 84.Perraudeau F, McMurdie P, Bullard J, Cheng A, Cutcliffe C, Deo A, Eid J, Gines J, Iyer M, Justice N, et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res Care. 2020;8(1):8. doi: 10.1136/bmjdrc-2020-001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’brien CE, Meier AK, Cernioglo K, Mitchell RD, Casaburi G, Frese SA, Henrick BM, Underwood MA, Smilowitz JT. Early probiotic supplementation with B. infantis in breastfed infants leads to persistent colonization at 1 year. Pediatr Res. 2022;91(3):627–636. doi: 10.1038/s41390-020-01350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frese SA, Hutton AA, Contreras LN, Shaw CA, Palumbo MC, Casaburi G, et al.Persistence of supplemented Bifidobacterium longum subsp. infantis EVC001 in breastfed infants mSphere 2017; 2. 10.1128/mSphere.00501-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS, Baccaglini L, Mohapatra A, Mohapatra SS, Misra PR, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548(7668):407–412. doi: 10.1038/nature23480. [DOI] [PubMed] [Google Scholar]
- 88.Cohen CR, Wierzbicki MR, French AL, Morris S, Newmann S, Reno H, Green L, Miller S, Powell J, Parks T, et al. Randomized trial of lactin-V to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382(20):1906–1915. doi: 10.1056/NEJMoa1915254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gardiner GE, Heinemann C, Bruce AW, Beuerman D, Reid G. Persistence of Lactobacillus fermentum RC-14 and Lactobacillus rhamnosus GR-1 but not L. rhamnosus GG in the human vagina as demonstrated by randomly amplified polymorphic DNA. Clin Diagn Lab Immunol. 2002;9(1):92–96. doi: 10.1128/CDLI.9.1.92-96.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Invernici MM, Salvador SL, Silva PHF, Soares MSM, Casarin R, Palioto DB, Souza SLS, Taba M, Novaes AB, Furlaneto FAC, et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: a randomized clinical trial. J Clin Periodontol. 2018;45(10):1198–1210. doi: 10.1111/jcpe.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Myles IA, Moore IN, Castillo CR, Datta SK. Differing virulence of healthy skin commensals in mouse models of infection. Front Cell Infect Microbiol. 2018;8:451. doi: 10.3389/fcimb.2018.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yelin I, Flett KB, Merakou C, Mehrotra P, Stam J, Snesrud E, Hinkle M, Lesho E, McGann P, McAdam AJ, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med. 2019;25(11):1728–1732. doi: 10.1038/s41591-019-0626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, Zur M, Regev-Lehavi D, Ben-Zeev Brik R, Federici S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174(6):1406–23 e16. doi: 10.1016/j.cell.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 94.Grazul H, Kanda LL, Gondek D. Impact of probiotic supplements on microbiome diversity following antibiotic treatment of mice. Gut Microbes. 2016;7(2):101–114. doi: 10.1080/19490976.2016.1138197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kabbani TA, Pallav K, Dowd SE, Villafuerte-Galvez J, Vanga RR, Castillo NE, Hansen J, Dennis M, Leffler DA, Kelly CP. Prospective randomized controlled study on the effects of Saccharomyces boulardii CNCM I-745 and amoxicillin-clavulanate or the combination on the gut microbiota of healthy volunteers. Gut Microbes. 2017;8(1):17–32. doi: 10.1080/19490976.2016.1267890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Castagnoli R, Pala F, Bosticardo M, Licari A, Delmonte OM, Villa A, Marseglia GL, Notarangelo LD. Gut microbiota–host interactions in inborn errors of immunity. Int J Mol Sci. 2021;22(3):1416. doi: 10.3390/ijms22031416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dorato MA, Buckley LA. Toxicology testing in drug discovery and development. Curr Protoc Toxicol. 2007;31(1). 19.1.1-.1.35. doi: 10.1002/0471141755.tx1901s31. [DOI] [PubMed] [Google Scholar]
- 98.Food and Drug Administration . MedWatch: the FDA safety information and adverse event reporting program. 2022. https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program
- 99.D’amico F, Parigi TL, Bonovas S, Peyrin-Biroulet L, Danese S. Long-term safety of approved biologics for ulcerative colitis. Expert Opin Drug Saf. 2020;19(7):807–816. doi: 10.1080/14740338.2020.1773430. [DOI] [PubMed] [Google Scholar]
- 100.Colerangle JB. Chapter 25 - preclinical development of nononcogenic drugs (small and large molecules). In: Faqi A editor. A comprehensive guide to toxicology in nonclinical drug development. Second. Boston: Academic Press; 2017. pp. 659–683. [Google Scholar]
- 101.U.S . Food and drug administration, center for food safety and applied nutrition, office of food additive safety. Redbook 2000: IV.C.5.a. Chronic Toxicity Studies with Rodents. 2007. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/redbook-2000-ivc5a-chronic-toxicity-studies-rodents
- 102.Veiga P, Suez J, Derrien M, Elinav E. Moving from probiotics to precision probiotics. Nat Microbiol. 2020;5(7):878–880. doi: 10.1038/s41564-020-0721-1. [DOI] [PubMed] [Google Scholar]
- 103.Bilal M, Ashraf S, Zhao X. Dietary component-induced inflammation and its amelioration by prebiotics, probiotics, and synbiotics. Front Nutr. 2022;9:931458. doi: 10.3389/fnut.2022.931458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanders ME, Akkermans LM, Haller D, Hammerman C, Heimbach J, Hormannsperger G, Huys G. Safety assessment of probiotics for human use. Gut Microbes. 2010;1(3):164–185. doi: 10.4161/gmic.1.3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kothari D, Patel S, Kim SK. Probiotic supplements might not be universally-effective and safe: a review. Biomed Pharmacother. 2019;111:537–547. doi: 10.1016/j.biopha.2018.12.104. [DOI] [PubMed] [Google Scholar]
- 106.Doern CD, Nguyen ST, Afolabi F, Burnham CA, Carroll KC. Probiotic-associated aspiration pneumonia due to Lactobacillus rhamnosus. J Clin Microbiol. 2014;52(8):3124–3126. doi: 10.1128/JCM.01065-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shinar E, Leitersdorf E, Yevin R. Lactobacillus plantarum endocarditis. Klin Wochenschr. 1984;62(24):1173–1174. doi: 10.1007/BF01712185. [DOI] [PubMed] [Google Scholar]
- 108.Ze-Ze L, Tenreiro R, Duarte A, Salgado MJ, Melo-Cristino J, Lito L, et al. Case of aortic endocarditis caused by Lactobacillus casei. J Med Microbiol. 2004;53(5):451–453. doi: 10.1099/jmm.0.05328-0. [DOI] [PubMed] [Google Scholar]
- 109.Franko B, Vaillant M, Recule C, Vautrin E, Brion JP, Pavese P. Lactobacillus paracasei endocarditis in a consumer of probiotics. Med Mal Infect. 2013;43(4):171–173. doi: 10.1016/j.medmal.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 110.Encarnacion CO, Loranger AM, Bharatkumar AG, Almassi GH. Bacterial endocarditis caused by lactobacillus acidophilus leading to rupture of sinus of valsalva aneurysm. Tex Heart Inst J. 2016;43(2):161–164. doi: 10.14503/THIJ-15-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boumis E, Capone A, Galati V, Venditti C, Petrosillo N. Probiotics and infective endocarditis in patients with hereditary hemorrhagic telangiectasia: a clinical case and a review of the literature. BMC Infect Dis. 2018;18(1):65. doi: 10.1186/s12879-018-2956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kunz AN, Noel JM, Fairchok MP. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J Pediatr Gastroenterol Nutr. 2004;38(4):457–458. doi: 10.1097/00005176-200404000-00017. [DOI] [PubMed] [Google Scholar]
- 113.Guenther K, Straube E, Pfister W, Guenther A, Huebler A. Sever sepsis after probiotic treatment with escherichia coli NISSLE 1917. Pediatr Infect Dis J. 2010;29(2):188–189. doi: 10.1097/INF.0b013e3181c36eb9. [DOI] [PubMed] [Google Scholar]
- 114.Vahabnezhad E, Mochon AB, Wozniak LJ, Ziring DA. Lactobacillus bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. J Clin Gastroenterol. 2013;47(5):437–439. doi: 10.1097/MCG.0b013e318279abf0. [DOI] [PubMed] [Google Scholar]
- 115.Zbinden A, Zbinden R, Berger C, Arlettaz R. Case series of bifidobacterium longum bacteremia in three preterm infants on probiotic therapy. Neonatology. 2015;107(1):56–59. doi: 10.1159/000367985. [DOI] [PubMed] [Google Scholar]
- 116.Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83(6):1256–1257. doi: 10.1093/ajcn/83.6.1256. [DOI] [PubMed] [Google Scholar]
- 117.Sheyholislami H, Connor KL. Are probiotics and prebiotics safe for use during pregnancy and lactation? A systematic review and meta-analysis. Nutrients. 2021;13(7):2382. doi: 10.3390/nu13072382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Elias J, Bozzo P, Einarson A. Are probiotics safe for use during pregnancy and lactation? Can Fam Physician. 2011;57:299–301. [PMC free article] [PubMed] [Google Scholar]
- 119.Jarde A, Lewis-Mikhael AM, Moayyedi P, Stearns JC, Collins SM, Beyene J, et al. Pregnancy outcomes in women taking probiotics or prebiotics: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):14. doi: 10.1186/s12884-017-1629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gille C, Boer B, Marschal M, Urschitz MS, Heinecke V, Hund V, Speidel S, Tarnow I, Mylonas I, Franz A, et al. Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial. Am J Obstet Gynecol. 2016;215(5):608 e1- e7 doi: 10.1016/j.ajog.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 121.Yang S, Reid G, Challis JRG, Gloor GB, Asztalos E, Money D, Seney S, Bocking AD. Effect of oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the vaginal microbiota, cytokines and chemokines in pregnant women. Nutrients. 2020;12(2):12. doi: 10.3390/nu12020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Davidson SJ, Barrett HL, Price SA, Callaway LK, Dekker Nitert M. Probiotics for preventing gestational diabetes. Cochrane Database Syst Rev. 2021;2021(4). CD009951. doi: 10.1002/14651858.CD009951.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fabian E, Kramer L, Siebert F, Högenauer C, Raggam RB, Wenzl H, Krejs G. D-lactic acidosis – case report and review of the literature. Z Fur Gastroenterol. 2017;55(01):75–82. doi: 10.1055/s-0042-117647. [DOI] [PubMed] [Google Scholar]
- 124.Munakata S, Arakawa C, Kohira R, Fujita Y, Fuchigami T, Mugishima H. A case of D-lactic acid encephalopathy associated with use of probiotics. Brain Dev. 2010;32(8):691–694. doi: 10.1016/j.braindev.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 125.Chp VDA, Jb VG, Szajewska H, Nd E, Hojsak I, Reid D, Shamir R. Probiotics for preterm infants: a strain-specific systematic review and network meta-analysis. J Pediatr Gastroenterol Nutr. 2018;67(1):103–122. doi: 10.1097/MPG.0000000000001897. [DOI] [PubMed] [Google Scholar]
- 126.Papagaroufalis K, Fotiou A, Egli D, Tran LA, Steenhout P. A randomized double blind controlled safety trial evaluating d-lactic acid production in healthy infants fed a Lactobacillus reuteri-containing formula. Nutr Metab Insights. 2014;7:19–27. doi: 10.4137/NMI.S14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lukasik J, Salminen S, Szajewska H. Rapid review shows that probiotics and fermented infant formulas do not cause d-lactic acidosis in healthy children. Acta Paediatr. 2018;107(8):1322–1326. doi: 10.1111/apa.14338. [DOI] [PubMed] [Google Scholar]
- 128.Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81(4):81. doi: 10.1128/MMBR.00036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.O’mahony SM, Clarke G, Dinan TG, Cryan JF. Early-life adversity and brain development: is the microbiome a missing piece of the puzzle? Neuroscience. 2017;342:37–54. doi: 10.1016/j.neuroscience.2015.09.068. [DOI] [PubMed] [Google Scholar]
- 130.Jacobs SE, Hickey L, Donath S, Opie GF, Anderson PJ, Garland SM, Cheong JLY. Probiotics, prematurity and neurodevelopment: follow-up of a randomised trial. BMJ Paediatr Open. 2017;1(1):e000176. doi: 10.1136/bmjpo-2017-000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stark CM, Susi A, Emerick J, Nylund CM. Antibiotic and acid-suppression medications during early childhood are associated with obesity. Gut. 2019;68(1):62–69. doi: 10.1136/gutjnl-2017-314971. [DOI] [PubMed] [Google Scholar]
- 132.Tun MH, Tun HM, Mahoney JJ, Konya TB, Guttman DS, Becker AB, Mandhane PJ, Turvey SE, Subbarao P, Sears MR, et al. Postnatal exposure to household disinfectants, infant gut microbiota and subsequent risk of overweight in children. CMAJ. 2018;190(37):E1097–107. doi: 10.1503/cmaj.170809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Karlsson Videhult F, Ohlund I, Stenlund H, Hernell O, West CE. Probiotics during weaning: a follow-up study on effects on body composition and metabolic markers at school age. Eur J Nutr. 2015;54(3):355–363. doi: 10.1007/s00394-014-0715-y. [DOI] [PubMed] [Google Scholar]
- 134.Lundelin K, Poussa T, Salminen S, Isolauri E. Long-term safety and efficacy of perinatal probiotic intervention: evidence from a follow-up study of four randomized, double-blind, placebo-controlled trials. Pediatr Allergy Immunol. 2017;28(2):170–175. doi: 10.1111/pai.12675. [DOI] [PubMed] [Google Scholar]
- 135.Jackson SA, Schoeni JL, Vegge C, Pane M, Stahl B, Bradley M, Goldman VS, Burguière P, Atwater JB, Sanders ME. Improving end-user trust in the quality of commercial probiotic products. Front Microbiol. 2019;10:739. doi: 10.3389/fmicb.2019.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.U.S. Food & Drug Administration . Current good manufacturing practice, hazard analysis, and risk-based preventive controls for human food. code of federal regulations title 21 2022; Part 117. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=117
- 137.U.S. Food & Drug Administration . Current good manufacturing practice in manufacturing, processing, packing, or holding of drugs. code of federal regulations title 21 2022; Part 210. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=210&showFR=1
- 138.Shane A, Preidis GA. 2023. Probiotics in the neonatal intensive care unit: a framework for optimizing product standards. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 139.Smith SM, Wang AT, Katz NP, McDermott MP, Burke LB, Coplan P, Gilron I, Hertz SH, Lin AH, Rappaport BA, et al. Adverse event assessment, analysis, and reporting in recent published analgesic clinical trials: aCTTION systematic review and recommendations. Pain. 2013;154(7):997–1008. doi: 10.1016/j.pain.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 140.Phillips R, Hazell L, Sauzet O, Cornelius V. Analysis and reporting of adverse events in randomised controlled trials: a review. BMJ Open. 2019;9(2):e024537. doi: 10.1136/bmjopen-2018-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cornelius VR, Sauzet O, Williams JE, Ayis S, Farquhar-Smith P, Ross JR, Branford RA, Peacock JL. Adverse event reporting in randomised controlled trials of neuropathic pain: considerations for future practice. Pain. 2013;154(2):213–220. doi: 10.1016/j.pain.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 142.Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA. 2001;285(4):437–443. doi: 10.1001/jama.285.4.437. [DOI] [PubMed] [Google Scholar]
- 143.Ioannidis JP, Evans SJ, Gotzsche PC, O’neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141(10):781–788. doi: 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]


