ABSTRACT
COVID-19 pandemic caused by SARS-CoV-2 infection has an impact on global public health and social economy. The emerging immune escape of SARS-CoV-2 variants pose great challenges to the development of vaccines based on original strains. The development of second-generation COVID-19 vaccines to induce immune responses with broad-spectrum protective effects is a matter of great urgency. Here, a prefusion-stabilized spike (S) trimer protein based on B.1.351 variant was expressed and prepared with CpG7909/aluminum hydroxide dual adjuvant to investigate the immunogenicity in mice. The results showed that the candidate vaccine could induce a significant receptor binding domain-specific antibody response and a substantial interferon-γ-mediated immune response. Furthermore, the candidate vaccine also elicited robust cross-neutralization against the pseudoviruses of the original strain, Beta variant, Delta variant and Omicron variant. The vaccine strategy of S-trimer protein formulated with CpG7909/aluminum hydroxide dual adjuvant may be considered a means to increase vaccine effectiveness against future variants.
KEYWORDS: SARS-CoV-2, vaccine, S-Trimer protein, CpG, aluminum hydroxide adjuvant, cross-neutralization
Introduction
More than 3 y into the COVID-19 pandemic, the world has recorded more than 656 million confirmed cases and more than 6.6 million deaths (https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—4-january-2023), which brought great challenges to the control of the epidemic.
Nowadays, COVID-19 vaccines including whole-inactivated virus vaccines, adenovirus-vector vaccines, mRNA vaccines and recombinant subunit protein vaccines were approved for use. With the continuous mutations and evolutions of the viruses, five predominant SARS-CoV-2 variants have been identified, which are Alpha (B.1. 1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1. 617.2) and Omicron (B.1. 1.529). The mutation sites of these variants are mainly in the receptor-binding domain (RBD) and N-terminal domain (NTD), resulting in more efficient entry of the virus into the host cell, increasing infectivity, and even evading immunity.1,2 More than 176 vaccines in clinical trials and 199 vaccines in preclinical researches have been reported in January 2023 (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines). Clinical studies observed that the cross-neutralization against the Omicron variant which induced by BNT162b2 and mRNA-1273 vaccines decreased by 30-fold after the second immunization, suggesting that the third dose of booster immunization was needed to provide neutralizing antibody response against Omicron variant.3,4 Moreover, the neutralizing antibody titers against Omicron in the serum of vaccinators or convalescent serum who infected with Alpha, Beta, Gamma or Delta SARS-CoV-2 variants decreased significantly, or even without neutralization.5 Therefore, it is necessary to update or develop a second-generation vaccine to prevent the infection and spread of various SARS-CoV-2 variants.6
SARS-CoV-2 invades into cells by engaging angiotensin-converting enzyme 2 (ACE2) on host cell surface with the viral spike glycoprotein (Spike, S),7 which is one of the predominant antigen targets for recombinant protein vaccines. Currently, most of the approved vaccines are based on prototype S protein of SARS-CoV-2. NVX-CoV2373, the leading recombinant protein vaccine based on prototype S protein, revealed a decreased efficacy from 95.6% to 49.4% against B.1.351 variant, and 59% against Omicron BA.4/BA.5.2,8 Briefly, B.1.351 variant as a dominant variant, characterized by three amino acid mutations in RBD (K417N, E484K, and N501Y) and a deletion in the NTD, decreased neutralization activity induced by non-B.1.351 SARS-CoV-2 infection or vaccination.9 Recent research found that Beta cross-reactive antibodies could neutralize not only Beta but also the original, Delta and Omicron variants.10 Moreover, mRNA vaccine based on Beta-Furin could trigger more broad and potent immune response to the genetically divergent SARS-CoV-2 variants than the Delta-Furin.11 In particular, some broad-spectrum cross-neutralizing activities against various SARS-CoV-2 variants induced by Beta protein were comparable to those by the bivalent vaccine SCTV01C.12 Thus, vaccines based on the Beta sequence showed promising cross-variant antibody titers in preclinical studies, which were valuable to be complemented by further studies of the immune response against recent and future SARS-CoV-2 variants, even though more vaccines currently focused on the Omicron variants.10,13
Additionally, to increase vaccine efficiency, some recombinant subunit vaccines based on the prefusion form of trimeric S protein antigen were required for novel adjuvants, such as Matrix-M,14 AS03,15 CpG and aluminum hydroxide (Al(OH)3) dual adjuvant system,16,17 etc. The additions of novel adjuvants in vaccines could induce more comprehensive immune responses, among which CpG and Al(OH)3 dual adjuvant vaccines could induce a stronger Th1-biased and more balanced Th1/Th2 cellular immune response, with high levels of neutralizing antibodies against SARS-CoV-2 variants,18–21 which was showed to be well tolerated and represent an effective strategy for the development of the second-generation vaccines.
To this end, in this study, S-Trimer protein based on the B.1.351 viral sequence was constructed and combined with CpG/Al (OH)3 dual adjuvant system as a candidate vaccine to evaluate the immunogenicity. The results elicited that the vaccine could activate humoral and interferon (IFN)-γ-mediated immune responses, and induce robust cross-neutralizing antibodies against the pseudoviruses of SARS-CoV-2 variants including Omicron. The research indicated that the highly immunogenic candidate vaccine could be used for sequential immunization or booster vaccine.
Materials and methods
Cells
ExpiCHO cells (Thermo Fisher) and HEK293-ACE2 cells (Vazyme Biotech Co.,Ltd) were cultured at 8% CO2 and 37°C.
Expression and identification of S-Trimer protein
To express the prefusion S protein, a plasmid was constructed to encode the ectodomain of B.1.351 SARS-CoV-2 S gene (MN908947.3, residues 12–1208), including the mutation sites D80A, D215G, 214del, 242del, 243del, K417N, E484K, N501Y, D614G and A701V. The original signal peptide of S protein was replaced by human antibody light chain signal peptide (MDMRVPAQLLGLLLLWLRGARC). The S1/S2 furin cleavage site 682-RRAR-685 was mutated into 682-QQAQ-685, with proline substitutions at residues 986 and 987. A C-terminal linker (GSG) and T4 fibritin trimerization motif (GYIPEAPRDGQAYVRKDGEWVLLSTFL) and His tag were synthesized and cloned into the mammalian expression vector pcDNA3.4. The S-Trimer protein was transfected and expressed by ExpiCHO cells, and then purified by affinity chromatography. S-Trimer protein was quantized by BCA (Thermo Fisher Scientific). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western Blot were run to check the purity of S-Trimer protein, and negative staining electron microscopy was performed for the trimeric conformation of S-Trimer protein.22
Receptor binding affinity of S-Trimer to ACE2
The binding affinity of S-Trimer to ACE2 was assessed by BIACORE. ACE2-Fc (10 μg/mL) was immobilized on Protein A biosensors, and S-Trimer antigen was continuously twofold diluted and then coupled with antibody to obtain real-time receptor-binding curves. Kinetic parameters (Kon and Koff) and affinities (KD) were analyzed by software.
Vaccine immunization
The mice study protocol (No. 2021003) was approved by the laboratory animal management committee and the laboratory animal ethics and welfare protection group of the Shanghai Institute of Biological Products.
Six- to eight-week-old BALB/c female mice (n = 5 per group) were intramuscularly vaccinated twice at D 0 and 21 with S-Trimer protein (1, 3, 10 μg) containing CpG 7909 (10, 150, 200, 300 μg) and Al(OH)3 (150 μg) adjuvant or non-adjuvant; control mice were mock-vaccinated (PBS, 50 μL per dose). Serum samples were collected on D 42 and stored at −20°C.
Enzyme-linked immunosorbent assay (ELISA)
To examine SARS-CoV-2 RBD-specific antibodies in the mouse sera, microplate was coated with 0.2 μg/well recombinant RBD protein (Sino Biological, Inc.) at 4°C overnight and blocked with 5% fetal bovine serum in PBST (0.05% Tween-20 in 1× PBS) for 1 h at 37°C. Serum samples were twofold diluted and applied to wells for 1 h at 37°C, followed by incubation with sheep anti-mouse IgG-peroxidase antibody for 1 h at 37°C. After incubation plates were added of 100 μL/well (3,3',5,5'-Tetramethylbenzidine), following the addition of 2 M H2SO4 to stop the reaction.23 The average value of the nagative group + 2×SD was used to set the antibody positive cutoff values.
Pseudovirus neutralization assays
The SARS-CoV-2 pseudovirus neutralization assay was conducted as previously described,24 with some modifications. First, the sample was inactivated at 56°C for 30 min and serially diluted (threefold), incubated with an equal volume of 100 TCID50 pseudovirus (wild-type original strain, B.1.617.2 variant strain, B.1.351 variant strain, B.1.1.529 variant strain) (Vazyme Biotech Co., Ltd) at 37°C for 1 h, along with virus-alone (positive control) and cell-alone (negative control). HEK293-ACE2 cells were added at a density of 30,000 cells/well. Following 48 h incubation, the cells were lysed and luciferase activity was determined by a Luciferase assay kit (PerkinElmer, 6066769). Neutralizing antibody titer of serum samples was determined by EC50, defined as the serum dilution at which the relative light units decreased by 50% compared to virus-alone control wells.
Enzyme-linked immunospot assay (ELISpot)
To detect antigen-specific T-cell responses, IFN-γ secreted by splenocytes was detected by ELISpot kits (Mabtech, 3321-3PT). Splenocytes from immunized mice were harvested on D 42 post immunizations. Splenocytes (5 × 105) were stimulated in vitro with 5 μg/mL of B.1.351 S-Trimer protein for 48 h incubation. Phorbol 12-myristate 13-acetate (PMA) was added to the positive control wells. Biotinylated detection antibodies from the ELISpot kits and SA-ALP/SA-HRP were added, and then 100 μL TMB color solution was added to each well-produced colored spots after 5–10 min incubation in the dark. Finally, the IFN-γ spot-forming cells were counted using an automatic ELISpot reader (CTL).25
Statistical analysis
GraphPad Prism 8.0 software was used for statistical analysis, one-way ANOVA test was used for comparison among multiple groups, and p < .05 was considered statistically significant.
Results
Expression and identification of S-Trimer antigen based on B.1.351 variant
Spike protein is the initial binding site when SARS-CoV-2 invades host cells through interaction with ACE2, which can be as a vaccine immunogen. In this study, B.1.351 S-Trimer protein antigen based on Beta variant was formed from the ectodomain of S protein 12–1208 and T4 fibritin trimerization motif, then expressed by ExpiCHO cell and purified by chromatography (Figure 1a). SDS-PAGE analysis displayed S protein monomer bands under reducing conditions, along with S protein monomer and trimer bands under non-reducing conditions, further confirmed by Western Blot analysis (Figure 1b).
Figure 1.
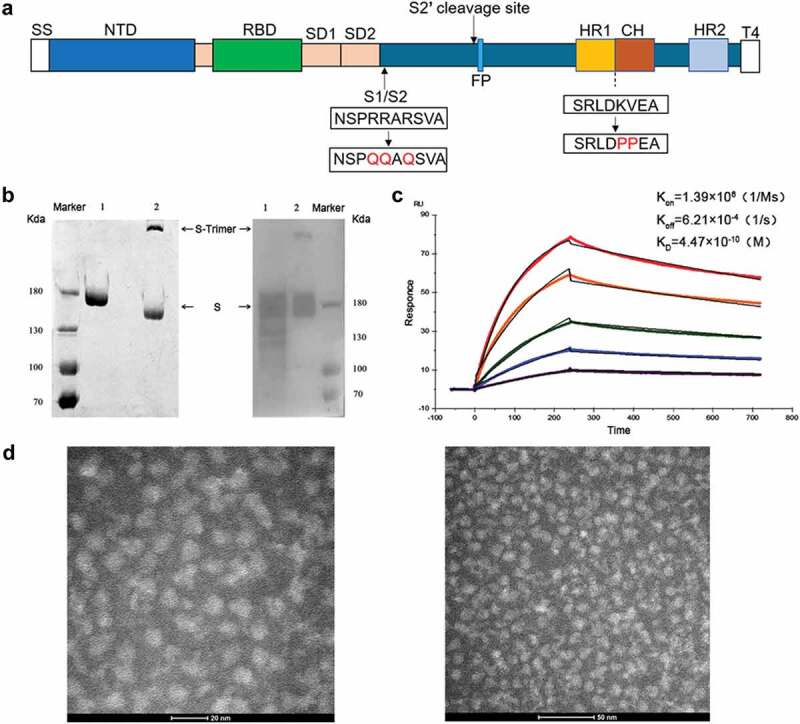
Design and characterization of S-Trimer antigen. (a) Schematic diagram of SARS-CoV-2 S-Trimer antigen protein. SS, signal sequence; NTD, N-terminal domain; RBD, receptor-binding domain; SD1, subdomain 1; SD2, subdomain 2; HR1, heptad repeat 1; CH, central helix; HR2, heptad repeat 2; T4: T4 fibritin trimerization motif. The recombinant SARS-CoV-2 spike antigen S-Trimer (pre-fusion S ectodomain with proline at residues 986 and 987 to retain S2 in the pre-fusion conformation, furin cleavage site replaced by “QQAQ,” and a C-terminal T4 fibritin trimerization motif added). (b) SDS-PAGE (Left) and Western blot (Right) analysis used to identify S-Trimer protein. The molecular weight standard is expressed as kDa on the left, lane 1 under reducing condition, and lane 2 under non-reducing condition. (c) The binding affinity (KD) of S-Trimer to ACE2 measured by BIACORE assay. (d) The structure of S-Trimer observed by transmission electron microscope.
The binding affinity of S-Trimer antigen to ACE2 receptor using BIACORE was shown to be approximately 4.47 × 10−10M (KD) (Figure 1c), which was about 10-fold higher than that of B.1.351 S-Trimer protein (5.2 × 10−9M) reported in previous result,26 indicating that S-Trimer here had better affinity. Negative staining electron microscopy confirmed that S-Trimer particles existed predominantly in a metastable, trimeric prefusion form (Figure 1d).
Humoral immune response induced by S-Trimer
Adjuvants could effectively enhance the immunogenicity of subunit proteins. To determine the humoral immune response induced by S-Trimer, mice were immunized twice with different doses (1, 3, 10 μg) of S-Trimer antigen formulated with CpG/Al(OH)3 dual adjuvant or without adjuvant. Moreover, the doses of CpG adjuvant were 10, 150, 200 and 300 μg, respectively, in order to evaluate the best formulation between antigen and adjuvant. Humoral immunogenicity analysis was conducted on D 42 blood samples based on ELISA and pseudovirus neutralization titers against the Beta variant.
RBD-specific IgG antibody titers induced by immunized mice were detected by ELISA. The results showed (Figure 2a) that all S-Trimer vaccine groups induced high titers of anti-RBD antibodies, and the antibody titers of antigen combined with different doses of CpG/Al(OH)3 dual adjuvant groups were statistically higher than those of S-Trimer antigen groups without adjuvant (p < .05). When the dose of CpG adjuvant was increased to 200 and 300 μg, the antibody titers induced by different doses of antigen formulated with CpG/Al(OH)3 was comparable, which was reach to 1.0 × 106 GMT (geometric mean titer). Indeed, 10 μg antigen formulated with 300 μg CpG/Al(OH)3 induced the highest RBD-specific antibody titers (2.0 × 106).
Figure 2.
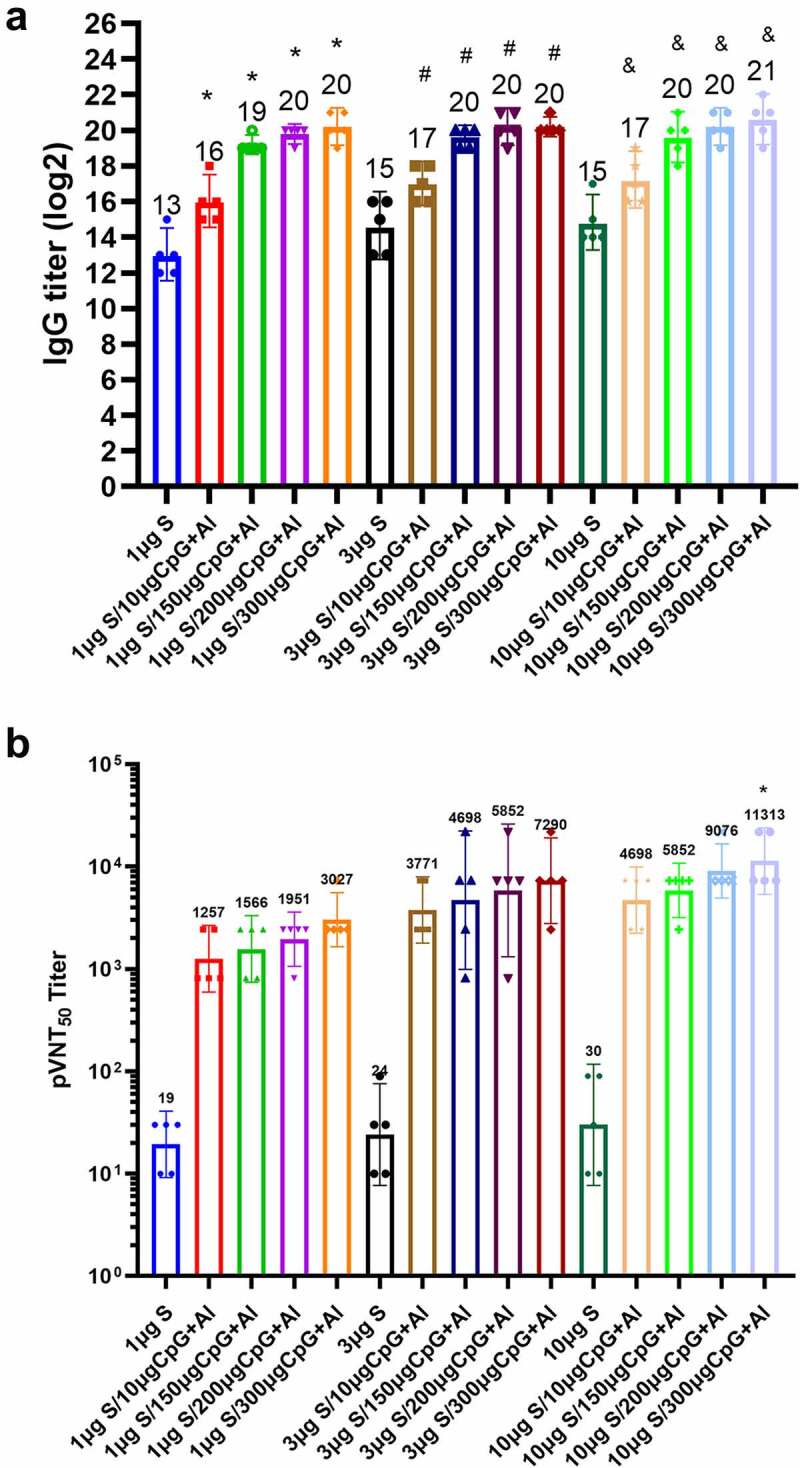
Humoral immune response induced by S-Trimer antigen in mice. BALB/c mice (n = 5/group) were immunized with various doses of S-Trimer that was non-adjuvanted or adjuvanted with CpG 7909 (10, 150, 200, 300 μg) and Al(OH)3 (150 μg) twice on D 0 and 21. The humoral immune responses on D 42 were analyzed. (a) The titers of RBD-specific IgG antibody in mice were determined by ELISA, * p < .05, were considered significant when compared to 1 μg S group; # p < .05, compared to 3 μg S group; & p < .05, compared to 10 μg S group. (B) The titers of pseudovirus neutralizing antibody against Beta variant in mice were detected by pseudovirus neutralization assay, * p < .05, which was statistically different from that of 10 μg S group. The number at the top of each bar represents the geometric mean titer (GMT).
When evaluating the neutralizing antibodies against Beta variant by pseudovirus neutralization assay, the magnitude of the response was dose-dependent, as evidenced by the observation that the addition of CpG/Al(OH)3 dual adjuvant to the antigen formulation significantly raised neutralizing antibody titers. The neutralizing antibody titers induced by 10 μg antigen formulated with 300 μg CpG/Al(OH)3 adjuvant had the highest titer of 11,313 GMT, which was statistically higher that those induced by 10 μg non-adjuvanted antigen (30) (p < .05). As expected, the neutralizing antibody titers of non-adjuvanted antigen were lower and below 30 GMT.
In addition, we noted that the neutralizing antibody titers induced by 1 μg antigen formulated with 10 μg CpG/Al(OH)3 (1257) adjuvant was significantly higher than that induced by 10 μg antigen group (30), which indicated that the addition of dual adjuvant could save at least 10-fold dose of the antigen, showing the antigen-sparing effect (Figure 2b).
Therefore, S-Trimer antigen combined with various doses of CpG/Al(OH)3 dual adjuvant could induce RBD-specific antibodies and neutralizing antibodies against Beta variant, to active humoral immune response.
IFN-γ-mediated immune response induced by S-Trimer
Cellular immune responses are essential to facilitate the production of antibodies and provide protection. Here, the levels of IFN-γ from splenocytes stimulated with S-Trimer protein were assessed by ELISpot assay (Figure 3). As expected for vaccines adjuvanted with CpG/Al(OH)3, IFN-γ secretions were induced, while the addition of CpG dose to formulation raised the increased levels of IFN-γ. Unexpectedly, when the dose of CpG adjuvant increased from 200 to 300 μg, the number of IFN-γ spots induced by 3 μg S-Trimer antigen combined with dual adjuvants are comparable to those of induced by 10 μg S-Trimer antigen combined with the same dual adjuvants, which was reach to 290 GMT, indicating that IFN-γ-mediated immune responses might reach a relative saturation state in mice.
Figure 3.
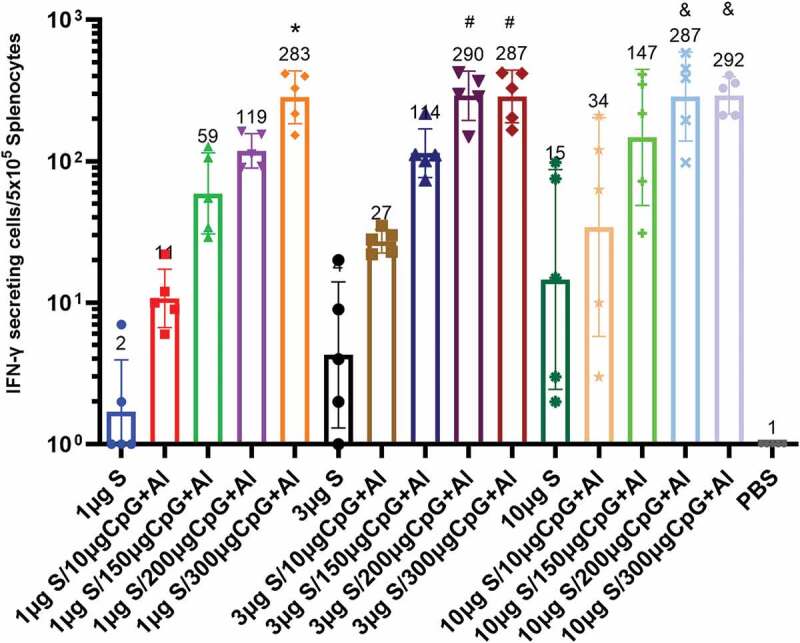
Cellular immune response induced by S-Trimer antigen in mice. BALB/c mice (n = 5/group) were immunized with various doses of S-Trimer that was non-adjuvanted or adjuvanted with CpG 7909 (10, 150, 200, 300 μg) and Al(OH)3 (150 μg) twice on D 0 and 21. The cellular immune responses on D 42 were assessed by ELISpot. *p < .05 were considered significant when compared to 1 μg S group; #p < .05, compared to 3 μg S group; & p < .05, compared to 10 μg S group. The number at the top of each bar represents the geometric mean titer (GMT).
Notably, S-Trimer antigen with various doses of CpG/Al(OH)3 dual adjuvant significantly induced IFN-γ-mediated immune response, indicating that the addition of adjuvants to the formulation could enhance the immune response effectively.
Robust cross-neutralizing antibodies induced by S-Trimer
To determine whether the vaccine can cross-reactive to different SARS-CoV-2 variants, we further evaluated the best immunogenic candidate vaccine, namely, the pseudoviruses neutralizing antibody of 10 μg S-Trimer antigen combined with 300 μg CpG/Al(OH)3 dual adjuvant against the other three variants: (1) the original SARS-CoV-2, (2) Delta (B.1.617.2) SARS-CoV-2 variant and (3) Omicron (B.1.1.529) SARS-CoV-2 variant.
Sera from mice vaccinated with the CpG/Al(OH)3 dual-adjuvanted vaccine candidate maintained the ability to neutralize pseudoviruses (Figure 4). The titers of pseudovirus neutralizing antibody against Delta variant was equivalent to that of Beta variant, while the neutralizing antibodies against original strain were about twofold lower than that of Beta variant, reaching 5852 GMT, and there was no statistical difference between the two groups. In addition, we did observe a 3.7-fold reduction in the GMTs against Omicron variant, while there was no statistical difference between the two groups. Although the pseudovirus neutralizing antibodies against the original strain and Omicron variant decreased, it was still at a high level, and the former was increased by 1.7-fold compared to human convalescent plasma (3415).26 The data indicated that S-Trimer antigen combined with CpG/Al(OH)3 dual adjuvant vaccine could offer robust antibodies that completely neutralize pseudoviruses of original strain, Beta variant, Delta variant and Omicron variant.
Figure 4.
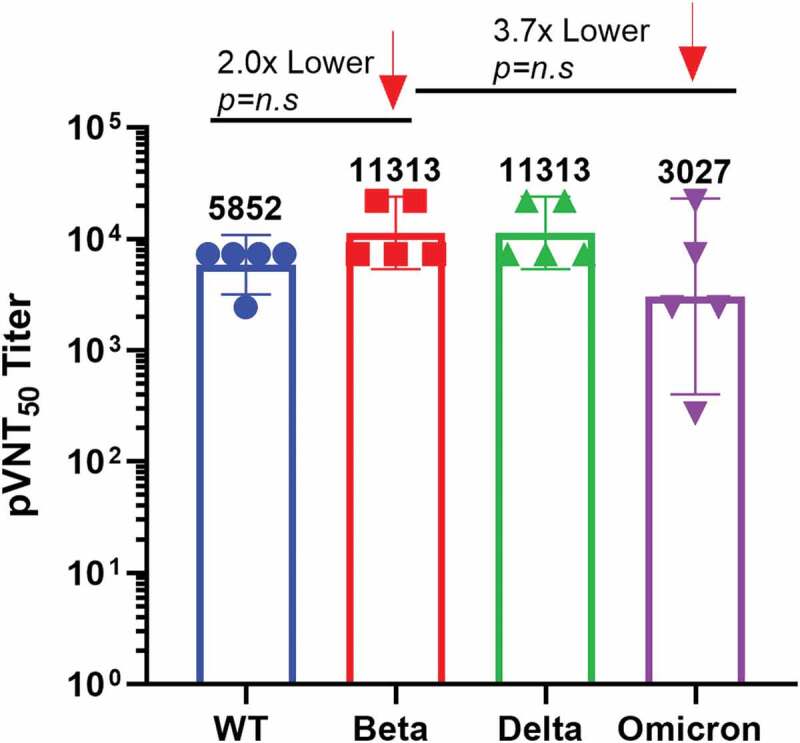
S-Trimer antigen induced robust cross-neutralizing antibodies against different SARS-CoV-2 variants. The pseudovirus neutralizing antibodies against the original strain, Beta variant, Delta variant and Omicron variant were detected in mice immunized with 10 μg S-Trimer antigen combined with 300 μg CpG/Al(OH)3 dual adjuvant vaccine on D 42. Pseudovirus neutralizing antibody data of different variants were compared to that of Beta variant. N = 5, each dot represents a single mouse, and the number above each strip represents geometric mean titer (GMT).
In summary, S-Trimer vaccine combined with CpG/Al(OH)3 dual adjuvant could induce humoral and IFN-γ-mediated immune responses in mice, and also elicit robust cross-neutralizing antibodies against various SARS-CoV-2 variants, which may be potentially suitable for candidate vaccines.
Discussion
Currently, several SARS-CoV-2 vaccines have been approved and proved to be effective in controlling COVID-19 pandemic, such as whole-inactivated virus vaccines, mRNA vaccines, adenovirus-vector vaccines and subunit recombinant vaccines. However, the constant mutations and immune escapes of SARS-CoV-2 variants, or the huge gaps in developing countries between the limited supply of vaccines and the huge population, demand the increases of the numbers of vaccinations or the developments of second-generation vaccines. Recombinant subunit vaccines have been proved to well tolerated and effective during this current COVID-19 outbreak.
In the current work, we elucidated that the recombinant B.1.351 S-Trimer protein combined with CpG 7909/Al(OH)3 dual adjuvant could induce strong humoral and cellular immune responses in mice, as well as broadly neutralize various SARS-CoV-2 pseudoviruses including Omicron variant, indicating strong immunogenicity. Phase II/III clinical trials showed that the overall effectiveness of two doses of SCB-2019 vaccine (S-Trimer combined with CpG 1018/Alum) was 67.2%, and the effective rate for moderate-to-severe COVID-19 was 83.7%, which could prevent severe cases 100% and provide protection against three SARS-CoV-2 variants, including Delta 78.7% and Gamma 91.8%. It is believed that the recombinant protein vaccine combined with CpG1018/Alum dual adjuvant could significantly provide protection on different variants.18
CpG and Al(OH)3 dual adjuvant system, as potent vaccine immune response enhancers, can induce more significant and lasting neutralizing antibody response. Oligonucleotide of CpG motif (CpG ODN) is an agonist of TLR9 and a potent vaccine adjuvant that produces Th1-biased cellular immune response.27,28 CpG 7909 and CpG 1018 belong to B-type CpG ODN, which activate MyD88, IRAK and TRAF6 signaling pathways by binding with endogenous TLR9 of B cells, dendritic cell or macrophages, induce proinflammatory immune response, and enhance adaptive immune response by stimulating human B cells and plasmacytoid dendritic cells.29,30 Some studies have found that the immune persistence of CpG7909-adjuvanted vaccine might be superior to CpG1018-adjuvanted.31,32 The candidate vaccine in this study focused on CpG7909 adjuvant, which could induce high level of IgG antibodies and promote the IFN-γ secretion of Th1-biased cellular immune response.
The emergence of numerous SARS-CoV-2 variants is one of the most important developments in the COVID-19 pandemic. Previous research showed that the prototype S-Trimer adjuvanted with CpG7909/Alum elicited neutralization titers against the original strain and cross-neutralizing antibodies against P.1, B.1.351 and B.1.617 variants.33,34 The modified B.1.351 S-Trimer formulated with CpG1018/Alum adjuvant also induced humoral immune responses and widely neutralized the original strain, B.1.1.7, B.1.351 and P.1 variants.26 The encouraging results of CpG 7909/Al(OH)3-adjuvanted S-Trimer in this study also warranted the neutralization titers against the original strain, B.1.617 variant, B.1.351 variant and especially the Omicron (B.1.1.529) variant, with the latter quickly becoming the most dominant SARS-CoV-2 lineage worldwide. Importantly, the CpG 7909/Al(OH)3-adjuvanted S-Trimer candidate vaccine based on the B.1.351 variant raised an apparent higher levels of cross-neutralization against the Omicron variant dominant globally in 2022. These findings appear to be consistent with that the infection on Beta (B.1.351) variant induced specific neutralizing antibodies against Beta variant and original strain, Delta variant and Omicron variant,10 and antibodies based on residue N417 of Beta variant could significantly crossly neutralize Omicron variant, Beta variant and Delta Plus variant.35 The chimeric nature of the Beta antigen sequence, which contained the mutations on RBD (K417N, E484K, N501Y, D614G) and on NTD, whereas the latter was co-dominant with RBD in vaccine-induced neutralizing antibodies,36 might explain that our modified B.1.351 S-Trimer subunit protein vaccine, could elicit anti-RBD antibodies targeting K417N and E484K mutations which neutralized B.1.351 and Omicron variants, and anti-NTD antibodies neutralized the rest of variants. Thus, the data suggested the modified B.1.351 S-Trimer subunit protein vaccine may be a broad-spectrum candidate vaccine for neutralizing various SARS-CoV-2 variants. Nevertheless, the human ACE2 transgenic mouse model and the live virus-based neutralization assay for the initial assessment of SARS-CoV-2 vaccine effectiveness, which may be a major limitation to the study, remain to be confirmed further.
Encouragingly, we evaluated the potential for “antigen and adjuvant sparing” dual effects in the current work. The neutralizing antibodies and IFN-γ-mediated immune responses induced by 1 μg S-Trimer combined with CpG/Al(OH)3 dual adjuvant were comparable to those induced by 10 μg S-Trimer antigen, indicating that the addition of adjuvant reduced at least 10-fold dosage of S-Trimer antigen and achieved antigen-sparing effect. By further comparison, a dose-dependent effect on the IFN-γ-mediated immune response was found in a certain adjuvant dose when under the same dose of antigen, while animals receiving 200 μg CpG-adjuvanted vaccine did not induce significantly higher immune response compared to animals receiving 300 μg CpG-adjuvanted doses. This might illustrate that the immune response induced by 200 μg CpG-adjuvanted vaccine reached a relative saturation state in mice, which provided a reference for optimizing the formula on the antigen and adjuvant. This is consistent with the observations that of receiving an adjuvanted boost (antigen plus CpG1018/Al), compared with animals receiving non-adjuvanted boost (antigen), the former did not induce significantly higher neutralizing antibody titers.26 Moreover, Trimer-Tag platform could improve the productivity of S-Trimer antigens and the potential of rapidly scale-up production to billions of doses,22 with the sparing of antigen and adjuvant, vaccines may be able to be prepared on a large scale, especially for low-income countries, which may be used as an additional tool against the SARS-CoV-2 virus that continues to spread.
Overall, the candidate vaccine here based on B.1.351 S-Trimer combined with CpG7909/Al(OH)3 dual adjuvant could elicit humoral and IFN-γ-mediated immune responses, and induce neutralizing antibodies against various SARS-CoV-2 variants, especially Omicron variant. These results warrant the further evaluation of booster strategy, also provide a reference for the development of a second-generation COVID-19 vaccine.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
All authors are employees of Shanghai Institute of Biological Products. The authors declare no conflict of interest.
Author contributions
Wrote the paper: Feixia Gao and Mei Zheng; Performed the experiments: Feixia Gao, Jiangfeng Fan and Yahong Ding; Analyzed the data: Xueying Liu and Min Zhang; Contributed reagents/materials: Xin Zhang and Jinrong Dong; Conceived and designed the experiments: Xu Zhou, Jian Luo and Xiuling Li.
Institutional review board statement
The animal study protocol was approved by the laboratory animal management committee and the laboratory animal ethics and welfare protection group of the Shanghai Institute of Biological Products (protocol code: 2021003).
References
- 1.Del RC, Omer SB, Malani PN.. Winter of omicron-the evolving COVID-19 pandemic. JAMA. 2022;327(4):319–8. doi: 10.1001/jama.2021.24315. [DOI] [PubMed] [Google Scholar]
- 2.Mohammadi M, Shayestehpour M, Mirzaei H.. The impact of spike mutated variants of SARS-CoV2 [alpha, beta, gamma, delta, and lambda] on the efficacy of subunit recombinant vaccines. Braz J Infect Dis. 2021;25(4):101606. doi: 10.1016/j.bjid.2021.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Beltran WF, St. Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, Berrios C, Ofoman O, Chang CC, Hauser BM, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185(3):457–66.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edara V, Manning KE, Ellis M, Lai L, Moore KM, Foster SL, Floyd K, Davis-Gardner ME, Mantus G, Nyhoff LE, et al. MRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep Med. 2022;3(2):100529. doi: 10.1016/j.xcrm.2022.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dejnirattisai W, Huo J, Zhou D, Zahradník J, Supasa P, Liu C, Duyvesteyn HME, Ginn HM, Mentzer AJ, Tuekprakhon A, et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185(3):467–84.e15. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382(21):1969–73. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 7.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci. 2020;117(21):202003138. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhiman JN, Richardson SI, Lambson BE, Kgagudi P, Mzindle N, Kaldine H, Crowther C, Gray G, Bekker L-G, Koen A, et al. Novavax NVX-COV2373 triggers neutralization of Omicron sub-lineages. Sci Rep. 2023;13(1):1222. doi: 10.1038/s41598-023-27698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He C, Yang J, He X, Hong W, Lei H, Chen Z, Shen G, Yang L, Li J, Wang Z, et al. A bivalent recombinant vaccine targeting the S1 protein induces neutralizing antibodies against both SARS-CoV-2 variants and wild-type of the virus. MedComm. 2020;2(3):430–41. doi: 10.1002/mco2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reincke SM, Yuan M, Kornau H-C, Corman VM, van Hoof S, Sánchez-Sendin E, Ramberger M, Yu W, Hua Y, Tien H, et al. SARS-CoV-2 beta variant infection elicits potent lineage-specific and cross-reactive antibodies. Science. 2022;375(6582):782–87. doi: 10.1126/science.abm5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Q, Zhao Y, Shaabani N, Lyu X, Powers C, Sun H, Cruz V, Stegman K, Xu J, Fossier A, et al. Chimeric mRNA-based COVID-19 vaccine induces protective immunity against Omicron and Delta variants. Mol Ther - Nucleic Acids. 2022;30:465–76. doi: 10.1016/j.omtn.2022.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Sun C, Ma J, Yu C, Kong D, Chen M, Liu X, Zhao D, Gao S, Kou S, et al. A bivalent COVID-19 vaccine based on alpha and beta variants elicits potent and broad immune responses in mice against SARS-CoV-2 variants. Vaccines (Basel). 2022;10(5):702. doi: 10.3390/vaccines10050702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ying B, Whitener B, VanBlargan LA, Hassan AO, Shrihari S, Liang C-Y, Karl CE, Mackin S, Chen RE, Kafai NM, et al. Protective activity of mRNA vaccines against ancestral and variant SARS-CoV-2 strains. Sci Transl Med. 2022;14(630):eabm3302. doi: 10.1126/scitranslmed.abm3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parums DV. Editorial: first approval of the protein-based adjuvanted nuvaxovid (NVX-Cov2373) Novavax vaccine for SARS-CoV-2 could increase vaccine uptake and provide immune protection from viral variants. Med Sci Monit. 2022;28:e936523. doi: 10.12659/MSM.936523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arunachalam PS, Walls AC, Golden N, Atyeo C, Fischinger S, Li C, Aye P, Navarro MJ, Lai L, Edara VV, et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature. 2021;594(7862):253–58. doi: 10.1038/s41586-021-03530-2. [DOI] [PubMed] [Google Scholar]
- 16.Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–32. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh S, Liu M-C, Chen Y-H, Lee W-S, Hwang S-J, Cheng S-H, Ko W-C, Hwang K-P, Wang N-C, Lee Y-L, et al. Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir Med. 2021;9(12):1396–406. doi: 10.1016/S2213-2600(21)00402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bravo L, Smolenov I, Han HH, Li P, Hosain R, Rockhold F, Clemens SAC, Roa C, Borja-Tabora C, Quinsaat A, et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2022;399(10323):461–72. doi: 10.1016/S0140-6736(22)00055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollet J, Strych U, Chen WH, Versteeg L, Keegan B, Zhan B, Wei J, Liu Z, Lee J, Kundu R, et al. Receptor-binding domain recombinant protein RBD219-N1C1 on alum-CpG induces broad protection against SARS-CoV-2 variants of concern. Vaccine. 2022;40(26):3655–3663. doi: 10.1016/j.vaccine.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo T, Lin M-Y, Coffman RL, Campbell JD, Traquina P, Lin Y-J, Liu LTC, Cheng J, Wu Y-C, Wu C-C, et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci Rep. 2020;10(1):20085. doi: 10.1038/s41598-020-77077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollet J, Chen W-H, Versteeg L, Keegan B, Zhan B, Wei J, Liu Z, Lee J, Kundu R, Adhikari R, et al. Sars‑coV-2 RBD219-N1C1: a yeast-expressed SARS-CoV-2 recombinant receptor-binding domain candidate vaccine stimulates virus neutralizing antibodies and T-cell immunity in mice. Hum Vaccines Immunother. 2021;17(8):2356–66. doi: 10.1080/21645515.2021.1901545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang JG, Su D, Song T-Z, Zeng Y, Huang W, Wu J, Xu R, Luo P, Yang X, Zhang X, et al. S-Trimer, a COVID-19 subunit vaccine candidate, induces protective immunity in nonhuman primates. Nat Commun. 2021;12(1). doi: 10.1038/s41467-021-21634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao F, Liu X, Dang Y, Duan P, Xu W, Zhang X, Wang S, Luo J, Li X. AddaVax-adjuvanted H5N8 inactivated vaccine induces robust humoral immune response against different clades of H5 viruses. Vaccine. 2022;10(10):1683. doi: 10.3390/vaccines10101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, Zhang L, Nie L, Qin H, Wang M, et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microbes Infect. 2020;9(1):680–86. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Huang X, Yuan L, Wang S, Zhang Y, Xiong H, Chen R, Ma J, Qi R, Nie M, et al. A recombinant spike protein subunit vaccine confers protective immunity against SARS-CoV-2 infection and transmission in hamsters. Sci Transl Med. 2021;13(606):eabg1143. doi: 10.1126/scitranslmed.abg1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su D, Li X, He C, Huang X, Chen M, Wang Q, Qin W, Liang Y, Xu R, Wu J, et al. Broad neutralization against SARS-CoV-2 variants induced by a modified B.1.351 protein-based COVID-19 vaccine candidate. bioRxiv. 2021. May 16:444369. doi: 10.1101/2021.05.16.444369. [DOI] [Google Scholar]
- 27.Shi S, Zhu H, Xia X, Liang Z, Ma X, Sun B. Vaccine adjuvants: understanding the structure and mechanism of adjuvanticity. Vaccine. 2019;37(24):3167–78. doi: 10.1016/j.vaccine.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 28.Kayraklioglu N, Horuluoglu B, Klinman DM. CpG oligonucleotides as vaccine adjuvants. Methods Mol Biol. 2021;2197:51–85. [DOI] [PubMed] [Google Scholar]
- 29.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5(6):471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 30.Scheiermann J, Klinman DM. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine. 2014;32(48):6377–89. doi: 10.1016/j.vaccine.2014.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halperin SA, Van Nest G, Smith B, Abtahi S, Whiley H, Eiden JJ. A phase I study of the safety and immunogenicity of recombinant hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide adjuvant. Vaccine. 2003;21(19–20):2461–67. doi: 10.1016/S0264-410X(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 32.Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, Cameron DW, Heathcote J. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24(6):693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Zhou C, An J, Song Y, Yu P, Li J, Gu C, Hu D, Jiang Y, Zhang L, et al. Development of recombinant COVID-19 vaccine based on CHO-produced, prefusion spike trimer and alum/CpG adjuvants. Vaccine. 2021;39(48):7001–11. doi: 10.1016/j.vaccine.2021.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Beltran WF, Lam EC, St. Denis K, Nitido AD, Garcia ZH, Hauser BM, Feldman J, Pavlovic MN, Gregory DJ, Poznansky MC, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184(9):2372–83.e9. doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moyo-Gwete T, Madzivhandila M, Mkhize NN, Kgagudi P, Ayres F, Lambson BE, Manamela NP, Richardson SI, Makhado Z, van der Mescht MA, et al. Shared N417-dependent epitope on the SARS-CoV-2 omicron, beta, and delta plus variants. J Virol. 2022;96(15):e0055822. doi: 10.1128/jvi.00558-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amanat F, Thapa M, Lei T, Sayed Ahmed SM, Adelsberg DC, Carreno JM, Strohmeier S, Schmitz AJ, Zafar S, Zhou JQ, Rijnink W, Alshammary H, Borcherding N, Reiche AG, Srivastava K, Sordillo EM, van Bakel H; Personalized Virology Initiative; Turner JS, Bajic G, Simon V, Ellebedy AH, Krammer F. The plasmablast response to SARS-CoV-2 mRNA vaccination is dominated by non-neutralizing antibodies and targets both the NTD and the RBD. medRxiv. 2021 May 1:2021 Mar 7:21253098. doi: 10.1101/2021.03.07.21253098 [DOI] [Google Scholar]


