Figure 4.
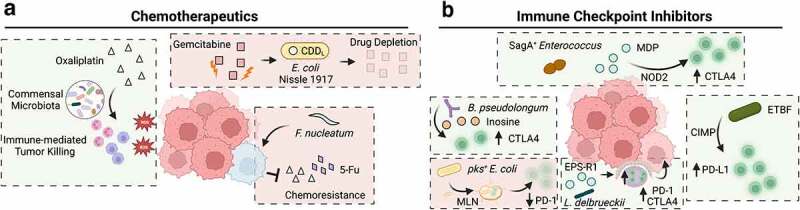
The impact of intestinal bacteria on colorectal cancer treatment. a) In mice harboring a commensal microbiota, neutrophils and macrophages invade tumors and produce reactive oxygen species (ROS), enhancing the tumor-killing effect of oxaliplatin. In mice administered antibiotics, the number of infiltrating immune cells and ROS-mediated cytotoxicity is reduced. In the presence of commensal bacteria (such as Escherichia coli Nissle 1917) harboring the long form of a cytidine deaminse (CDDL), gemcitabine levels are reduced after degradation by this bacterial enzyme, resulting in a reduced therapeutic response. Similarly, infection with Fusobacterium nucleatum confers resistance to oxaliplatin and 5-Fu by downregulating miRNAs that suppress autophagy and survival signaling. b) Several bacterial species have been linked to enhanced immunotherapy response in murine models of CRC or MC-38 xenografts. The species Bifidobacterium longum produces a metabolite inosine, which activates tumor-infiltrating T cells and exacerbates tumor killing after anti-CTLA4 treatment. Enterococcus spp. harboring the secreted antigen A (sagA) gene generate high levels of muramyl dipeptide (MDP) that activates nucleotide-binding oligomerization domain-containing protein 2 (NOD2) signaling pathways in colonic epithelial cells that drives immune recruitment and synergizes with anti-CTLA4 treatment. In a murine model of CpG island methylator phenotype (CIMP) CRC, infection with Enterotoxigenic Bacteroides fragilis promotes the recruitment of interferon gamma (IFNγ)-producing CD8+ T cells to enhance anti-PD-1 efficacy. Lactobacillus delbrueckii subsp. bulgaricus exopolysaccharides (EPS-R1) promote the activation of CCR6+CD8+ T cells in intestinal Peyer’s patches, as well as the number of IFNγ producing CD8+ tumor infiltrating cells, promoting the efficacy of anti-PD-1 and anti-CTLA4 treatment. Conversely, pks+ E. coli can migrate to mesenteric lymph nodes (MLN) and reduce systemic levels of CD3+ and CD8+ T cells, as well as the number of these cells observed in invasive tumor margins and reduces anti-PD-1 treatment efficacy.
