Figure 5. AcrIC4 occludes the PAM site but makes different Cascade contacts than AcrIF2.
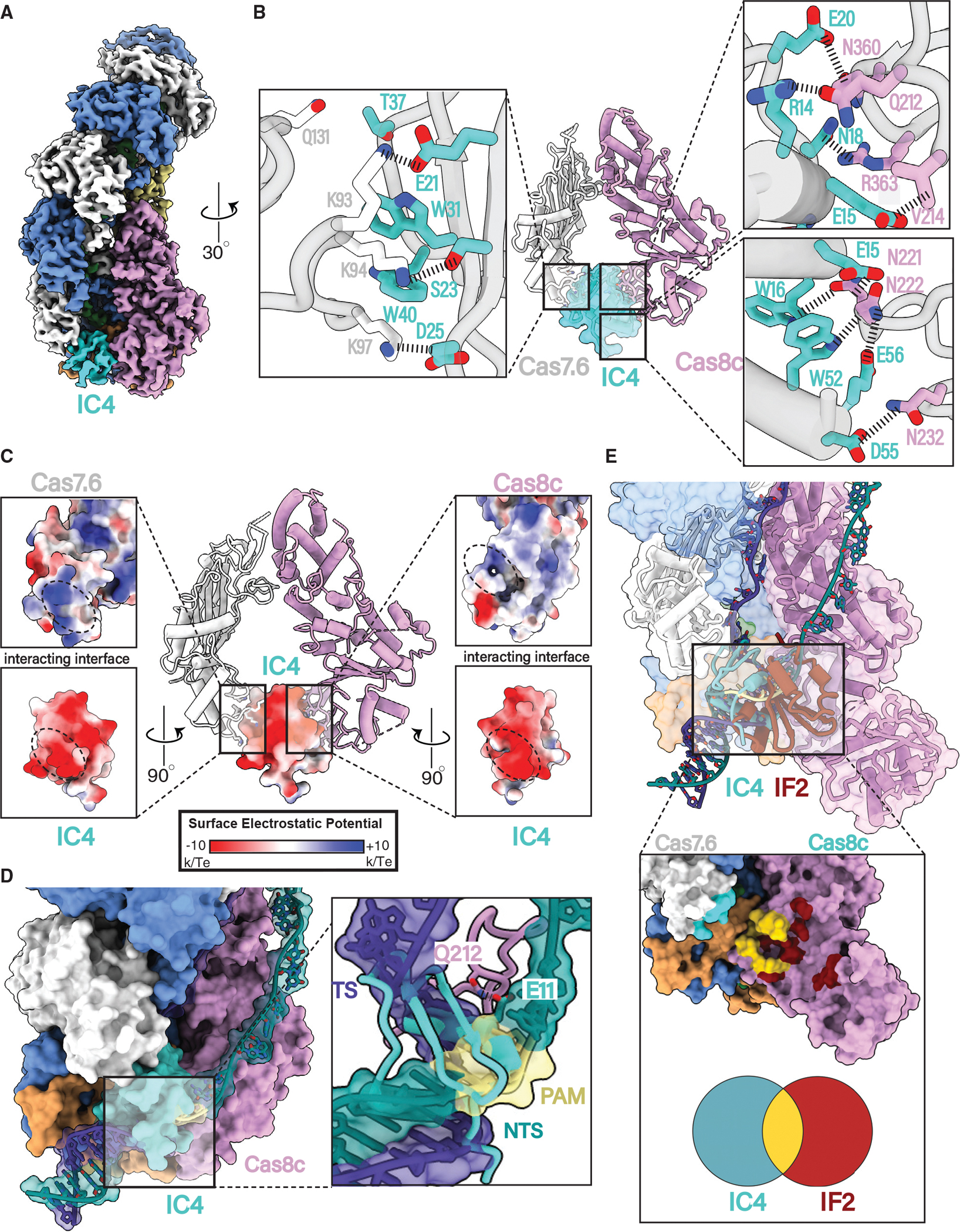
(A) 3.1 Å resolution cryoelectron structure of the type I-C Cascade bound to AcrIC4.
(B) AcrIC4 interacts with both Cas8c and Cas7c through a large network of non-specific interactions.
(C) AcrIC4 is entirely negatively charged and wedged between positively charged Cas8c and Cas7.6 surfaces.
(D) Structural superposition of the type I-C dsDNA-bound model shows severe clashing between PAM residues and AcrIC4.
(E) Overlay of the IF2 and IC4 binding sites demonstrates a partially overlapping interface at the PAM site.
