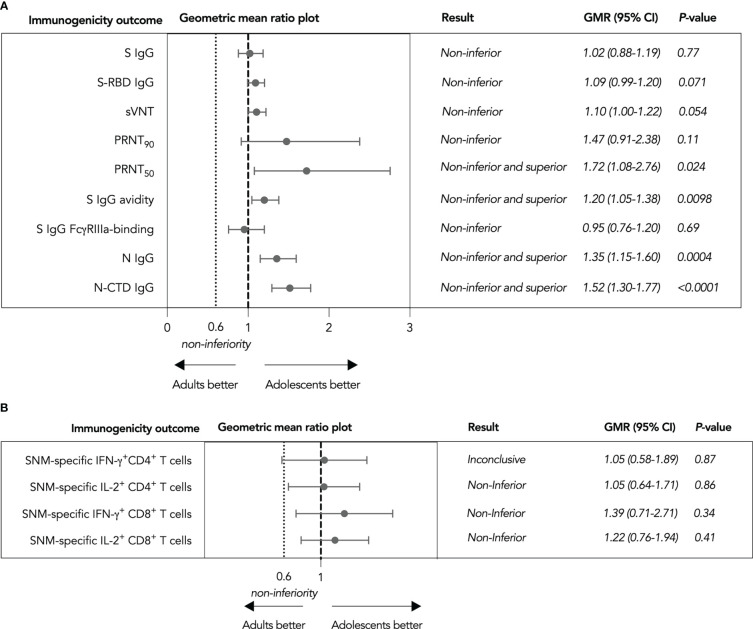
Figure 2.
Non-inferiority hypothesis testing of humoral and cellular immunogenicity outcomes against wild-type SARS-CoV-2 after the third dose of CoronaVac in evaluable analysis population. (A) Non-inferiority testing of SARS-CoV-2 Spike (S) IgG, S-receptor binding domain (S-RBD) IgG, surrogate virus neutralization test (sVNT), plaque reduction neutralization test (PRNT), S IgG avidity, S IgG Fcγ receptor IIIa (FcγRIIIa)-binding, Nucleocapsid (N) IgG, and N-C-terminal domain (N-CTD) IgG (B Non-inferiority testing of total S, N and Membrane (M) protein-specific interferon-γ (IFN-γ)+ and interleukin-2 (IL-2)+ CD4+ and CD8+ T cells Geometric mean ratios (GMR) and two-tailed 95% confidence intervals (CI) were plotted.