Abstract
Introduction
Previous studies suggest diagnostic testing characteristics (i.e. variations in clinical specimens and diagnostic tests) can contribute to underestimation of RSV disease burden. We aimed to improve the understanding of RSV hospitalisation burden in older adults (aged ≥ 65 years) in high-income countries through adjusting for case under-ascertainment.
Methods
We conducted a systematic review to include data on RSV-associated acute respiratory infection (ARI) hospitalisation burden in older adults in high-income countries. To adjust for case under-ascertainment, we developed a two-step framework that incorporated empirical data on the RSV detection proportion of different clinical specimens and testing approaches as well as their statistical uncertainty. We estimated the unadjusted and adjusted RSV-associated hospitalisation burden through multilevel random-effects meta-analysis. We further explored RSV-associated in-hospital mortality burden.
Results
We included 12 studies with eligible RSV hospitalisation burden data. We estimated that pooled unadjusted hospitalisation rate was 157 per 100,000 (95% CI 98–252) for adults aged ≥ 65 years; the rate was adjusted to 347 per 100,000 (203–595) after accounting for under-ascertainment. The adjusted rate could be translated into 787,000 (460,000–1,347,000) RSV-associated hospitalisations in high-income countries in 2019, which was about 2.2 times the unadjusted estimate. Stratified analysis by age group showed that the adjusted rate increased with age, from 231 per 100,000 in adults aged 65–74 years to 692 per 100,000 in adults aged > 85 years. The in-hospital case fatality ratio of RSV was 6.1% (3.3–11.0) and the total RSV-associated in-hospital deaths in high-income countries in 2019 could be between 22,000 and 47,000.
Conclusion
This study improves the understanding of RSV-associated hospitalisation burden in older adults and shows that the true RSV-associated hospitalisation burden could be 2.2 times what was reported in existing studies. This study has implications for calculating the benefit of interventions to treat and prevent RSV-associated disease.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00792-3.
Keywords: Respiratory syncytial virus, Older adults, Hospitalisation, Disease burden, Under-ascertainment, Sensitivity, Mortality
Key Summary Points
| Why carry out this study? |
| Diagnostic testing characteristics (i.e. variations in clinical specimens and diagnostic tests) can contribute to underestimation of RSV disease burden. |
| Existing reports on RSV disease burden did not fully account for case under-ascertainment. |
| We aimed to improve the understanding of RSV hospitalisation burden in older adults from high-income countries by accounting for case under-ascertainment. |
| What was learned from this study? |
| The true RSV-associated hospitalisation burden could be 2.2 times what was reported in existing studies without adjustment for case under-ascertainment. |
| Accounting for case under-ascertainment has implications for calculating the benefit of interventions to treat and prevent RSV disease. |
Introduction
Respiratory syncytial virus (RSV) is a major cause of acute respiratory infections (ARI) in older adults over 65 years. Although there are no currently licensed RSV vaccines or specific antiviral therapies, several RSV vaccine candidates for adults are at late stage of clinical development [1]. Of these, three promising candidate vaccines are reported to have met the primary efficacy endpoint against RSV-associated ARI in older adults following a phase 3 clinical trial: RSVpreF (Pfizer) [2], RSVPreF3 OA (GSK) [3], and mRNA-1345 (Moderna) [4]. Moreover, RSV therapeutics are also being evaluated. Therefore, it is important to understand the disease burden of RSV in older adults to inform public health and clinical decision-making.
There have been several reports of the global and regional RSV disease burden in older adults [5–8]. However, these reports were based on studies with various RSV diagnostic testing characteristics (i.e. clinical specimens and diagnostic tests) that had suboptimal sensitivity, resulting in underestimation of the true RSV disease burden. For example, a study that compared different diagnostic tests of RSV among adults found that only half of the RSV cases confirmed by reverse transcription polymerase chain reaction (RT-PCR) had a positive viral culture result [9]. Furthermore, a growing body of literature recently summarised in a systematic review and meta-analysis suggests that use of nasopharyngeal (NP) or nasal swabs alone to identify RSV infection yields downwardly biased estimates of RSV disease rates [10]. Compared to NP/nasal swab alone, RSV detection increased by 52% when adding RT-PCR of sputum, 28% when adding RT-PCR of oropharyngeal swabs, and 42% when adding serology testing of paired specimens. On this basis, a recently published systematic review and meta-analysis of studies adjusted for case under-ascertainment using a correction factor of 1.5 to reflect the additionally identified RSV cases by adding paired serology or sputum [11]. However, this analysis did not include studies using diagnostics other than RT-PCR or serology (e.g. rapid antigen test), which could also be used for adjusting for case under-ascertainment [12]; the adjustment did not consider the diagnostic value of a third (or more) diagnostic test or specimen, in addition to paired serology and sputum. Moreover, no studies have evaluated the benefit of adding saliva, which has been shown to be a sensitive specimen type for viral respiratory testing [13, 14].
It is thus necessary to explicitly adjust for the under-ascertainment of RSV detection associated with the selection of clinical specimens and testing methods when estimating RSV disease burden, including accounting for the synergistic effects on RSV detection of adding multiple specimen types.
In this study, we aimed to improve the understanding of RSV-associated ARI hospitalisation burden in older adults in high-income countries by adjusting for case under-ascertainment due to diagnostic testing characteristics. This was achieved by developing a framework that could incorporate an innate uncertainty range around the levels of under-ascertainment when estimating the adjusted RSV hospitalisation burden.
Methods
Systematic Literature Review
We conducted a systematic literature review (registered with PROSPERO: CRD42022311521) to identify studies reporting RSV-associated ARI hospitalisation burden in adults aged 60 years or above in high-income countries (defined by the 2019 World Bank Income Classification [15], a complete list in Supplementary Material). We searched six databases (MEDLINE, Embase, Global Health, CINAHL, Web of Science, and Global Index Medicus) for relevant studies published between 1 January 1996 and 16 December 2021, using tailored search strategies (Supplementary Material). The detailed inclusion and exclusion criteria are as follows.
Inclusion Criteria
Reporting on RSV-infected adults aged 60 years or above as primary infection with ARI (defined as onset of any respiratory symptoms within 10 days) necessitating hospital admission; AND
Reporting on RSV in the overall older adult population, i.e. not limited to persons with specific comorbidities like asthma, coronary artery disease, obesity, diabetes, or COPD; AND
With available data for at least 12 consecutive months, except where RSV seasonality has been well established (e.g. temperate regions where surveillance is typically conducted during the autumn–winter months [16]); AND
Reporting hospital admission rates when population denominator (e.g. hospital catchment population) is available. Alternatively, the proportion of RSV positivity in all hospitalised ARI testing for RSV is reported.
Exclusion Criteria
Reporting RSV infection not as the primary outcome; OR
Case definition not clearly defined; OR
Case definition narrower than ARI (e.g. influenza-like illness, severe acute respiratory infection [SARI], or community-acquired pneumonia); OR
Focusing on specific populations such as patients with cancer, haematological patients or transplant patients; OR [15]
Estimates based on modelling approaches (e.g. excess hospitalisation); OR
Not reporting in English language.
A self-designed data collection template was used for data collection. The template collected study-level information including location and country, study period, case definition, clinical specimen, RSV testing method, and reported estimates by age group. As substantial variations in age group reporting were anticipated, we chose not to restrict to certain age groups when extracting the data; this means that all age groups that fell into the broad category of 60 years or above would be considered for inclusion. Data screening and extraction was conducted independently by DK and jointly by BW and PW, with any disagreements resolved mutually among members of the review team.
For all included studies, quality assessment was conducted independently by two reviewers using the Joanna Briggs Institute (JBI) critical appraisal tools according to the study design [17]. The detailed list of questions is provided in the quality assessment section of the Supplementary Material. For each study, we calculated the percentage of questions that received an answer of “yes” (indicating high quality) in all questions, excluding questions that were not applicable. Studies with 80% or more of the questions receiving “yes” were considered high quality.
Adjustment Ratio for Under-Ascertainment
We considered both the adjustment for clinical specimen and for testing approaches in this analysis, following a two-step framework.
As the first step, we considered testing approaches while conditioning on clinical specimen (i.e. NP swabs for all approaches except for serology), assuming that a combined testing by PCR, culture and paired serology would be the gold standard for confirmation of RSV infections. We calculated the proportion of detection by each possible combination of testing approach(es) compared with the gold standard, as reported by Falsey and colleagues [9] (assuming that any positives by any of the three methods—PCR, culture and paired serology—were true positives); as a result, the detection proportion should range from 0 (lowest sensitivity) to 1 (perfect sensitivity). We also calculated the proportion of detection by rapid antigen testing compared with the gold standard through a systematic review of RSV diagnostic accuracy comparison [10]. As the second step, we focused on adjusting for clinical specimen conditioning on testing method. We assumed that a combination of sputum, saliva, and nasopharyngeal specimens testing through PCR plus paired serology would be the gold standard. The detection proportion was calculated on the basis of a prospective multi-specimen study that found a 1.6-fold increase in RSV detection adding saliva and sputum specimens to nasopharyngeal swab [18]. For both steps, we incorporated the innate statistical uncertainty around the testing accuracy studies through a Bayesian approach and sampled 1000 times from the posterior beta distribution of detection proportion based on data and assumed non-informative prior distribution (i.e. a beta(1,1) distribution); detailed posterior distributions used for generating sample detection proportions are in Supplementary Material.
Finally, we calculated the products of the two detection proportions obtained from the two steps and calculated the reciprocals of the products to obtain the adjustment ratio for under-ascertainment, which should be above 1. The list of the adjustment ratios used in the study can be found in Supplementary Material.
Hospitalisation Burden Estimation
The primary outcome measure was the pooled RSV-associated ARI hospitalisation rate in high-income countries, adjusting and not adjusting for under-ascertainment (as stated above). The secondary outcome measures were the pooled proportion of RSV in all-cause ARI hospitalisations (adjusting and not adjusting for under-ascertainment) and in-hospital case fatality ratio (CFR) of RSV-associated ARI hospitalisations (not adjusting for under-ascertainment since no studies determined the CFR for ARI hospitalisations that were missed by RSV testing).
To generate the adjusted pooled RSV-associated ARI hospitalisation rate, we first inflated the RSV detection for each study on the basis of its clinical specimen and testing approach by applying the adjustment ratios as obtained above. We then conducted random-effects meta-analysis that accounted for clustering induced by multiple data points from the same study (e.g. when a study reported RSV-associated ARI hospitalisation rates separately for each study year). The unadjusted meta-estimate was generated using the same random-effects meta-analysis without applying any adjustment ratios. Moreover, we applied the 2019 population data to the meta-estimated rates to obtain the number of RSV-associated ARI hospitalisations in high-income countries in 2019.
To generate the adjusted pooled proportion of RSV in all-cause ARI hospitalisations, we first inflated the RSV detection for each study on the basis of its clinical specimen and testing approach by applying the adjustment ratios, same to above. Then we conducted generalised linear mixed-effects model (GLMM) to synthesise the proportion of RSV in ARI hospitalisations; the use of GLMM was based on the anticipation that some studies had limited sample size (i.e. the number of RSV positives) [19]. The unadjusted meta-estimate was generated using GLMM without applying any adjustment ratios.
We conducted GLMM meta-analysis to generate the pooled CFR of RSV-associated ARI hospitalisations. The CFR was then applied to the number of RSV-ARI hospitalisations to obtain the number of RSV-associated ARI in-hospital deaths. As the CFR for those infected with RSV but not testing positive for RSV (i.e. false negatives) was unknown, the CFR was applied to both the adjusted and unadjusted numbers of RSV-ARI hospitalisations, with the former assuming identical CFR to those testing positive for RSV and the latter assuming zero CFR. We hypothesised that RSV false negatives tended to have lower viral load and less disease severity; as a result, the two approaches for estimating RSV-associated ARI in-hospital deaths jointly provided a plausible range of true RSV-associated ARI in-hospital deaths among older adults in high-income countries.
To avoid inflation of the statistical uncertainty range induced by multiple-step modelling, where applicable, we generated 1000 Monte Carlo samples from normal distribution at each step; conducted all calculations per sample; and calculated the 95% confidence interval by taking the 2.5th and 97.5th percentiles of the 1000 samples [19].
We conducted the analysis above by each of the age groups as they were originally reported in the literature. As the majority of included studies defined older adults as those aged 65 years or above (rather than 60 years or above as used in the literature search), we considered 65 years or above as the main age group for reporting and included other finer age bands as stratified analysis.
Sensitivity Analysis
We conducted a series of sensitivity analyses to check the robustness of our main outcome measure (i.e. RSV-associated ARI hospitalisation burden). First, we considered only prospective studies in the analysis given that testing practice was expected to be more consistently applied in prospective studies. Second, we retained only high-quality studies as defined above in the analysis. Third, we applied the proportion of RSV testing positives in all-cause ARI hospitalisations obtained in this analysis to our previous estimates of total number of all-cause ARI hospitalisations for the year 2019 to obtain the number of RSV-associated ARI hospitalisations in high-income countries [6].
Statistical Software and Ethics
All statistical analyses were conducted using the R software (version 4.1.2). This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Therefore, no ethical approval is required.
Results
Characteristics of Included Studies
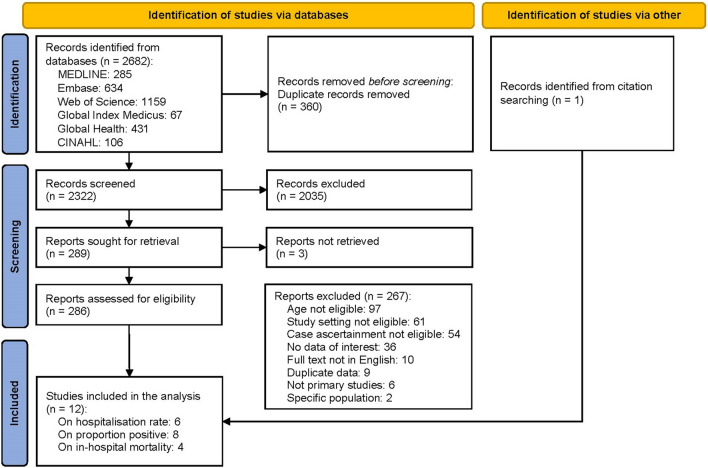
Through the systematic literature search, we identified a total of 2322 records after removal of duplicates, of which 11 studies were deemed eligible; in addition, we included one study through citation searching, which brought the total number of included studies to 12 (Fig. 1). The complete list of included studies is in the Supplementary Material. Of these studies, eight were conducted in the USA; two were conducted in Finland; and two were conducted in New Zealand. The median number of RSV seasons reported was 3 years. Half of the included studies were assessed as high quality on the basis of the quality assessment results (details in the quality assessment results section of the Supplementary Material).
Fig. 1.
PRSIMA flow chart showing selection process of published literature
Hospitalisation Rate
Six studies reported RSV-associated ARI hospitalisation rate estimates [20–25]; details of the hospitalisation rates in each individual study are in Supplementary Material. Without adjustment for under-ascertainment, the pooled annual RSV-associated ARI hospitalisation rate in those aged 65 years or above was 157 per 100,000 (95% CI 98–252), corresponding to 356,000 (222,000–572,000) RSV-associated ARI hospitalisations in all high-income countries in 2019. The adjusted annual RSV-associated ARI hospitalisation rate in persons aged 65 years or above was 347 per 100,000 (203–595), corresponding to 787,000 (460,000–1,347,000) RSV-associated ARI hospitalisations in 2019, approximately 2.2 times the unadjusted estimate. Sensitivity analyses that only included prospective studies and only included high-quality studies did not yield statistically different estimates from the main analysis (Supplementary Material). Within the 65 years or above category, there was an increasing trend in RSV-associated ARI hospitalisation rate with age for both unadjusted and adjusted estimates, although this finding was based on a limited number of studies (i.e. one or two studies in each finer age band). Overall, heterogeneity in RSV-associated ARI hospitalisation rate across studies was high; compared with unadjusted estimates, the adjusted estimates showed reduced heterogeneity (from 95% to 94% for 65 years or above; from 58% to 47% for 85 years or above (Table 1).
Table 1.
Annual RSV-associated ARI hospitalisation rate and number (in 2019) in high-income countries
| Age group | Number of studies (data points) | Adjusted for under-ascertainment | Hospitalisation rate per 100,000 (95% CI) | Number of hospitalisations (95% CI) | I2 |
|---|---|---|---|---|---|
| 65 years or above | 6 (14) | Unadjusted | 157 ( 98–252) | 356,000 (222,000–572,000) | 95% |
| 6 (14) | Adjusted | 347 (203–595) | 787,000 (460,000–1,347,000) | 94% | |
| 65–74 years | 1 (6) | Unadjusted | 105 (91–120) | 129,000 (112,000–148,000) | NE |
| 1 (6) | Adjusted | 231 (169–315) | 285,000 (209,000–388,000) | NE | |
| 75–84 years | 1 (6) | Unadjusted | 200 (173–230) | 144,000 (125,000–166,000) | NE |
| 1 (6) | Adjusted | 441 (323–601) | 317,000 (233,000–433,000) | NE | |
| 85 years or above | 2 (10) | Unadjusted | 312 (209–467) | 98,000 ( 65,000–146,000) | 58% |
| 2 (10) | Adjusted | 692 (430–1114) | 216,000 (134,000–348,000) | 47% |
CI confidence interval, NE not estimable
Proportion of RSV Detection
Eight studies reported the proportion of RSV detection in all-cause ARI hospitalisation [20, 22, 23, 25–29]; seven of them reported the proportion for the age group of 65 years or above. Without adjustment for under-ascertainment, RSV was found in 7.9% (5.4–11.5) of older adults aged 65 years or above hospitalised for ARI; this proportion increased to 16.5% (10.3–25.2) after accounting for under-ascertainment. Through applying the proportion of RSV to our previous estimate of ARI hospitalisations in adults aged 65 years or above (3,368,000 in high-income countries in 2019) [30], the number of RSV-associated ARI hospitalisations was estimated to be 267,000 (182,000–386,000) and 554,000 (347,000–849,000), without and with adjustment for under-ascertainment, respectively (Table 2).
Table 2.
Proportion of RSV positives in ARI hospitalisations and estimated number of RSV-associated ARI hospitalisation (in 2019) in high-income countries through external data source
| Age group | Number of studies | Adjusted for under-ascertainment | Proportion in % of RSV positives (95% CI) | Number of hospitalisations (95% CI) estimated by applying RSV proportion* |
|---|---|---|---|---|
| 65 years or above | 7 | Unadjusted | 7.9 ( 5.4–11.5) | 267,000 (182,000–386,000) |
| 7 | Adjusted | 16.5 (10.3–25.2) | 554,000 (347,000–849,000) |
RSV respiratory syncytial virus, CI confidence interval
*This was calculated by applying the proportion of RSV positives obtained in this study to the estimate of all-cause ARI hospitalisations in adults aged 65 years or above (3,072,000 in high-income countries in 2015, converted to 3,368,000 in 2019) from Shi and colleagues [30]
In-Hospital Mortality
Four studies reported the in-hospital CFR of laboratory-confirmed RSV-associated ARI hospitalisation [26, 27, 31, 32]. Based on two studies each, the pooled CFR for 65 years or above and 60 years or above was 6.1% (3.3–11.0) and 5.3% (3.9–7.3), respectively (Table 3). Based on the two assumptions regarding the in-hospital CFR of RSV false negatives, the number of in-hospital deaths of RSV-associated ARI in adults aged 65 years or above in high-income countries in 2019 could lie between 22,000 (i.e. if CFR for false negatives is zero) and 47,000 (i.e. if CFR for false negatives was the same as those detected by available study testing).
Table 3.
RSV-associated ARI in-hospital case fatality ratio and deaths in high-income countries
| Age group | Number of studies | CFR (95% CI) of ARI hospitalisations testing positive for RSV | Number of in-hospital deaths (95% CI) under two assumptions in 2019 | |
|---|---|---|---|---|
| Under-ascertained RSV cases having same CFR as those testing positive for RSV | Under-ascertained RSV cases having zero CFR | |||
| 65 years or above | 2 | 6.1 (3.3–11.0) | 47,000 (16,000–148,000) | 22,000 (7,000–66,000) |
| 60 years or above | 2 | 5.3 (3.9–7.3) | NE* | NE* |
RSV respiratory syncytial virus, CFR case fatality ratio, CI confidence interval, NE not estimable
*Not estimable because of the absence of studies reporting RSV-associated ARI hospitalisation in those aged 60 years or above
Discussion
In this study, we explicitly accounted for the potential case under-ascertainment of RSV-associated ARI hospitalisations related to clinical specimens and testing approaches by developing a two-step framework that incorporated empirical data on the detection proportion of different clinical specimens and testing approaches as well as their statistical uncertainty. We estimated that the true RSV-associated hospitalisation burden was likely to be substantially higher—at least 2.2 times what was reported in existing studies. The annual RSV-associated ARI hospitalisation rate in those aged 65 years or above in high-income countries was estimated as 347 (95% CI 203–595) per 100,000, corresponding to 787,000 (460,000–1,347,000) hospitalisations in 2019. The CFR of RSV-associated ARI hospitalisation was 6.1% (3.3–11.0) for 65 years or above; depending on the true CFR of RSV false negatives, the number of in-hospital RSV-associated ARI deaths could lie between 22,000 and 47,000 in 2019. These estimates will assist with calculating the benefit of therapeutic and preventive (e.g. vaccinations) interventions for RSV in older adults.
The unadjusted estimate of RSV-associated ARI hospitalisation rate in this study was broadly consistent with those reported in existing systematic reviews and analyses [5, 11, 30] despite differences in eligibility criteria for input studies and data (Table 4). In addition, results from the sensitivity analyses in this study yielded similar estimates to the main analysis. These consistencies support the robustness of the estimate in this study. To our knowledge, the only existing analysis accounting for case under-ascertainment was the study by McLaughlin and colleagues [11] that reported rates of medically attended RSV (including hospitalisation) in US adults. McLaughlin and colleagues calculated the relative increase in RSV detection based on adding one additional clinical specimen (serum or sputum) to nasal or nasopharyngeal swab (tested by PCR) alone, and then applied the median detection ratio for adjusting for under-ascertainment. Different from the McLaughlin study, our study considers both diagnostic testing and clinical specimen for the adjustment; regarding clinical specimen, the synergistic effects of using a combination of multiple clinical specimens were considered (rather than two in the McLaughlin study). Regarding the testing approach, we adjusted for under-ascertainment from using rapid antigen testing rather than PCR testing. These differences explained the higher adjusted hospitalisation rate reported in this study (347 per 100,000; 203–595) than the McLaughlin study (267 per 100,000; 228–306). Furthermore, the McLaughlin study did not consider the innate statistical uncertainty around the detection ratio per se (i.e. only the median ratio was applied), which resulted in artificially narrower confidence intervals of the estimate; by contrast, we explicitly accounted for such uncertainty through a Bayesian approach, which better reflected the precision of the estimate.
Table 4.
Comparisons of RSV-associated ARI hospitalisation rates between this study and previous systematic reviews
| Study | Age group | Adjusted for under-ascertainment | Estimates (95% CI), per 100,000 | Key study characteristics for comparison |
|---|---|---|---|---|
| This study | 65 years or above | No | 157 (98–252) | Strictly limited to ARI |
| This study | 65 years or above | Yes | 347 (203–595) | Strictly limited to ARI |
| Shi et al. 2020 [30] | 65 years or above | No | 100 (50–210) | A wide range of case definitions allowed (which could be narrower than ARI) |
| Savic et al. 2022 [5] | 60 years or above | No | 145 ( 94–224) | Hospitalisation attack rate reported (rather than annual hospitalisation rate) |
| McLaughlin et al. 2022 [11] | 65 years or above | No | 178 (152–204) | Limited to the USA; including all RSV-associated hospitalisations; not excluding modelling studies |
| McLaughlin et al. 2022 [11] | 65 years or above | Partly* | 267 (228–306) | Limited to the USA; including all RSV-associated hospitalisations; not excluding modelling studies; limited to studies using PCR or serology |
RSV respiratory syncytial virus, ARI acute respiratory infection, PCR polymerase chain reaction
*The adjustment was made by applying an RSV detection ratio that quantified the improved diagnostic yield after adding a second specimen type (serology or sputum); only the median detection ratio was used for the adjustment (i.e. not accounting for the innate statistical uncertainty around the detection ratio)
Previous studies showed inconsistent estimates of in-hospital CFR of RSV-associated ARI hospitalisation in older adults residing in high-income countries. An analysis by Shi and colleagues [30] estimated a pooled CFR of 1.6% (0.7–3.8) in those aged 65 years or above in industrialised countries whereas a more recent analysis by Savic and colleagues [5] reported a much higher CFR of 7.1% (5.4–9.4) in those aged 60 years or above. Such inconsistency was a result of limited evidence from published literature as well as varied eligibility criteria and methodologies adopted in these analyses. Taking the case definition for ARI as an example, studies that included only severe ARI cases might report a higher CFR than those focusing on the full spectrum of ARI; including studies reporting only severe ARI in-hospital CFR would result in an overestimation. In our study, we applied a stricter case definition where any subsets of ARI (e.g. influenza-like illness or SARI) were not considered eligible. This could explain the lower CFR estimate for those aged 60 years or above in this study (5.3%; 3.9–7.3) than the Savic study. It should be noted that all existing CFR estimates for RSV-associated hospitalisations were based on those that had tested positive for RSV; the CFR for persons with false negative test results (i.e. those missed by certain testing approaches or specimens) remains unknown. On the basis of the hypothesis that the CFR for RSV false negatives should be lower than that for RSV true positives (due to possibly lower viral loads), we estimated that the total number of RSV-associated ARI in-hospital deaths could lie between 22,000 (assuming zero CFR of RSV false negatives) and 47,000 (assuming same CFR of RSV false negatives as RSV true positives) in 2019. A recent meta-analysis [33] with no restrictions to locations reported an in-hospital CFR of 10% for pneumococcal community-acquired pneumonia and 14% for all-cause community-acquired pneumonia in older adults of 65 years or above so it is likely that our RSV estimates are conservative.
Compared to previous analyses of this type, we made extensive efforts to account for heterogeneity across different studies, which included the restriction of case definition to ARI and the adjustment for varied RSV diagnostic testing characteristics. Despite these additional precautions, we acknowledge that heterogeneity was still substantial across included studies. The observed heterogeneity was likely a result of varied study populations, healthcare-seeking behaviours, admission criteria, and RSV testing criteria. In addition, we did not stratify the estimation by comorbidity status as this required the total number of populations with comorbidity (i.e. population at risk), which was not available; previous studies from the USA showed that older adults with underlying medical conditions were more likely to be hospitalised for RSV infections than those without [21, 34]. Although stratified analysis was conducted by finer age group within the age category of 65 years or above, the estimate was based only on one to two studies for each age group and should be interpreted with caution; nonetheless, the stratified analysis confirmed the increasing trend over age in the RSV-associated ARI hospitalisation rate among the older adults. Similarly, for the in-hospital RSV mortality burden, the CFR estimates had wider confidence intervals due to data scarcity; further evidence is warranted to consolidate the RSV mortality burden estimate given as it is influential in health economic assessment for future RSV immunisation strategy. Finally, all data on RSV hospitalisation burden included in this study were before the COVID-19 pandemic; the epidemiology of RSV changed after the onset of the COVID-19 pandemic with loss of RSV immunity across all age groups [35, 36]. As a result, the RSV hospitalisation burden in older adults may change in the coming years.
Conclusion
This study improves the understanding of the hospitalisation burden of RSV-associated ARI in older adults in high-income countries and provides important evidence for decision-making for RSV prevention and control. Most importantly, we showed that the true RSV-associated hospitalisation burden was likely substantially underestimated—an estimated 2.2-fold in our study—compared to what previous studies have reported after explicitly adjusting for the varied RSV diagnostic testing characteristics related to clinical specimens and testing approaches. This framework of adjustment has potential application for the wider field of disease burden estimation, where the impact of suboptimal diagnostics is expected to be substantial.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Pfizer funded this study and the journal’s Rapid Service fee.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article.
Author Contributions
Harish Nair, Bradford Gessner, and Elizabeth Begier conceptualised the study with inputs from You Li. Durga Kulkarni, Pia Wahi-Singh, and Bhanu Wahi-Singh led the data collection. You Li led the data analysis and interpretation with inputs from Elizabeth Begier and Harish Nair. You Li wrote the first draft of the report. All authors reviewed the draft for intellectual content and approved the final report.
Disclosures
You Li reports consulting fees from Pfizer, related to the submitted work; and grants from Wellcome Trust and GSK, outside the submitted work. Durga Kulkarni reports consulting fees from Pfizer, related to the submitted work; and consulting fees from Pfizer outside the submitted work. Elizabeth Begier and Bradford Gessner are employees and shareholders of Pfizer. Harish Nair reports consulting fees from Pfizer, related to the submitted work; and grants from the Innovative Medicines Initiative outside the submitted work; consulting fees from the Gates Foundation, Pfizer, and Sanofi; honoraria from AbbVie; support from Sanofi for attending meetings; and participation on advisory boards from GSK, Merck, Pfizer, Sanofi, Icosavax, Janssen, Novavax, Reviral, Resvinet, and WHO outside the submitted work. All other authors declared that they have no competing interests.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Therefore, no ethical approval is required.
Data Availability
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.PATH. RSV vaccine and mAb snapshot. https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/. Accessed 30 Nov 2022.
- 2.Pfizer announces positive top-line data from phase 3 trial of older adults for its bivalent respiratory syncytial virus (RSV) vaccine candidate. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-phase-3-trial-older. Accessed 30 Nov 2022.
- 3.GSK’s older adult respiratory syncytial virus (RSV) vaccine candidate shows 94.1% reduction in severe RSV disease and overall vaccine efficacy of 82.6% in pivotal trial. https://www.gsk.com/en-gb/media/press-releases/gsk-s-older-adult-respiratory-syncytial-virus-rsv-vaccine-candidate/. Accessed 30 Nov 2022.
- 4.Moderna. Moderna announces MRNA-1345, an investigational respiratory syncytial virus (RSV) vaccine, has met primary efficacy endpoints in phase 3 trial in older adults. https://investors.modernatx.com/news/news-details/2023/Moderna-Announces-mRNA-1345-an-Investigational-Respiratory-Syncytial-Virus-RSV-Vaccine-Has-Met-Primary-Efficacy-Endpoints-in-Phase-3-Trial-in-Older-Adults/default.aspx. Accessed 31 Jan 2023.
- 5.Savic M, Penders Y, Shi T, Branche A, Pirçon JY. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: a systematic literature review and meta-analysis. Influenza Respir Viruses. 2022 doi: 10.1111/irv.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi T, Denouel A, Tietjen AK, et al. Global and regional burden of hospital admissions for pneumonia in older adults: a systematic review and meta-analysis. J Infect Dis. 2019;222:S570–S576. doi: 10.1093/infdis/jiz053. [DOI] [PubMed] [Google Scholar]
- 7.Tin Tin Htar M, Yerramalla MS, Moïsi JC, Swerdlow DL. The burden of respiratory syncytial virus in adults: a systematic review and meta-analysis. Epidemiol Infect. 2020;148:48. doi: 10.1017/S0950268820000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen-Van-Tam JS, O'Leary M, Martin ET, et al. Burden of respiratory syncytial virus infection in older and high-risk adults: a systematic review and meta-analysis of the evidence from developed countries. Eur Respir Rev. 2022;31:220105. doi: 10.1183/16000617.0105-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falsey AR, Formica MA, Walsh EE. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol. 2002;40:817–820. doi: 10.1128/JCM.40.3.817-820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onwuchekwa C, Moreo LM, Menon S, et al. Underascertainment of respiratory syncytial virus infection in adults due to diagnostic testing limitations: a systematic literature review and meta-analysis. J Infect Dis. 2023 doi: 10.1093/infdis/jiad012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin JM, Khan F, Begier E, Swerdlow DL, Jodar L, Falsey AR. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open forum infectious diseases. Oxford: Oxford University Press; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chartrand C, Tremblay N, Renaud C, Papenburg J. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: systematic review and meta-analysis. J Clin Microbiol. 2015;53:3738–3749. doi: 10.1128/JCM.01816-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyllie AL, Fournier J, Casanovas-Massana A, et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020;383:1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez J, Carrico R, Wilde A. Adding sputum and saliva to nasopharyngeal swab samples for PCV detection of respiratory syncytial virus in adults hospitalized with acute respiratory illness may double case detection. IDWeek. Oxford: Oxford University Press; 2022. [Google Scholar]
- 15.The World Bank. World Bank country and lending groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519. Accessed 30 Nov 2022.
- 16.Li Y, Reeves RM, Wang X, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health. 2019;7:e1031–e1045. doi: 10.1016/S2214-109X(19)30264-5. [DOI] [PubMed] [Google Scholar]
- 17.JBI. Critical appraisal tools. https://jbi.global/critical-appraisal-tools. Accessed 30 Nov 2022.
- 18.Ramirez JA, Carrico R, Wilde AM, et al. Adding sputum and saliva to nasopharyngeal swab samples for PCR detection of respiratory syncytial virus in adults hospitalized with acute respiratory illness may double case detection. Open Forum Infect Dis. 2022 doi: 10.1093/ofid/ofac492.449. [DOI] [Google Scholar]
- 19.Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auvinen R, Syrjänen R, Ollgren J, Nohynek H, Skogberg K. Clinical characteristics and population-based attack rates of respiratory syncytial virus versus influenza hospitalizations among adults—an observational study. Influenza Other Respir Viruses. 2022;16:276–288. doi: 10.1111/irv.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branche AR, Saiman L, Walsh EE, et al. Incidence of respiratory syncytial virus infection among hospitalized adults, 2017–2020. Clin Infect Dis. 2022;74:1004–1011. doi: 10.1093/cid/ciab595. [DOI] [PubMed] [Google Scholar]
- 22.Nolen LD, Seeman S, Desnoyers C, et al. Respiratory syncytial virus and influenza hospitalizations in Alaska native adults. J Clin Virol. 2020;127:104347. doi: 10.1016/j.jcv.2020.104347. [DOI] [PubMed] [Google Scholar]
- 23.Prasad N, Newbern EC, Trenholme AA, et al. The health and economic burden of respiratory syncytial virus associated hospitalizations in adults. PLoS ONE. 2020;15:e0234235. doi: 10.1371/journal.pone.0234235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widmer K, Griffin MR, Zhu Y, Williams JV, Talbot HK. Respiratory syncytial virus- and human metapneumovirus-associated emergency department and hospital burden in adults. Influenza Other Respir Viruses. 2014;8:347–352. doi: 10.1111/irv.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206:56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aronen M, Viikari L, Kohonen I, et al. Respiratory tract virus infections in the elderly with pneumonia. BMC Geriatr. 2019;19:111. doi: 10.1186/s12877-019-1125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 28.Malosh RE, Martin ET, Callear AP, et al. Respiratory syncytial virus hospitalization in middle-aged and older adults. J Clin Virol. 2017;96:37–43. doi: 10.1016/j.jcv.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasad N, Walker TA, Waite B, et al. Respiratory syncytial virus-associated hospitalizations among adults with chronic medical conditions. Clin Infect Dis. 2021;73:e158–e163. doi: 10.1093/cid/ciaa730. [DOI] [PubMed] [Google Scholar]
- 30.Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis. 2020;222:S577–S583. doi: 10.1093/infdis/jiz059. [DOI] [PubMed] [Google Scholar]
- 31.Belongia EA, King JP, Kieke BA, et al. Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults ≥60 years old. Open Forum Infect Dis. 2018;5:ofy316. doi: 10.1093/ofid/ofy316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng HF, Sy LS, Ackerson B, et al. Severe morbidity and short- and mid- to long-term mortality in older adults hospitalized with respiratory syncytial virus infection. J Infect Dis. 2020;222:1298–1310. doi: 10.1093/infdis/jiaa361. [DOI] [PubMed] [Google Scholar]
- 33.Polkowska-Kramek A, Theilacker C, Sato R, et al. Mortality risk associated with pneumococcal community acquired pneumonia in adults: results of systematic literature review and meta-analysis. In: International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-12), Toronto, Canada, 19–23 June 2022.
- 34.Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V. Estimates of hospitalization attributable to influenza and RSV in the US during 1997–2009, by age and risk status. BMC Public Health. 2017;17:271. doi: 10.1186/s12889-017-4177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.den Hartog G, van Kasteren PB, Schepp RM, Teirlinck AC, van der Klis FRM, van Binnendijk RS. Decline of RSV-specific antibodies during the COVID-19 pandemic. Lancet Infect Dis. 2023;23:23–25. doi: 10.1016/S1473-3099(22)00763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reicherz F, Xu RY, Abu-Raya B, et al. Waning immunity against respiratory syncytial virus during the coronavirus disease 2019 pandemic. J Infect Dis. 2022;226:2064–2068. doi: 10.1093/infdis/jiac192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.