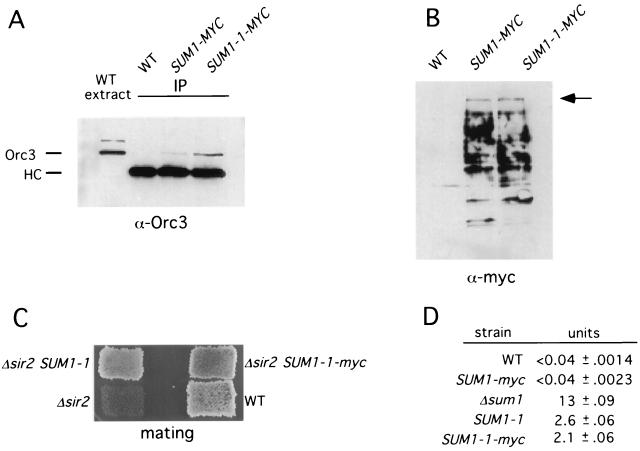
FIG. 4.
Sum1-1 coimmunoprecipitates with Orc3. (A) IP analysis with Orc3. Strains YRH15 (WT), YRH20 (SUM1-myc), and YRH21 (SUM1-1-myc) were transformed with plasmid pRH34 (HMR in pRS426). Extracts were prepared and proteins were immunoprecipitated using anti-myc antibodies. Following SDS-polyacrylamide gel electrophoresis, the precipitates were analyzed by immunoblotting and probed with an anti-Orc3 antibody. A portion of the wild-type extract from YRH15 (with plasmid pRH34) was also run on the same gel to show the position of Orc3 as well as a slower-migrating protein that interacts with the Orc3 antibody (20). HC, heavy chain of IgG. (B) A portion (5%) of the precipitated material used in panel A was analyzed by immunoblotting with anti-myc antibodies. The Sum1-myc and Sum1-1–myc proteins always show multiple bands following immunoprecipitation, presumably due to proteolysis. The arrow points to the position of the full-length proteins. (C) Sum1-1–myc functions in vivo. Sum1-1–myc silencing in Δsir2 strains was measured by a patch-mating assay with WT (W303-1b), Δsir2 (YRH15), Δsir2 SUM1-1 (YRH34), or Δsir2 SUM1-1-myc (YRH36) strains. (D) Sum1-myc functions in vivo. Strains were transformed with plasmid pJX43 (SMK1 MSE-lacZ) and grown to early log phase in SC −Ura medium. β-Galactosidase activity was determined for three independent transformants for each strain. WT (YRH15), SUM1-myc (YRH20), Δsum1 (JXY3), SUM1-1 (YRH34), and SUM1-1-myc (YRH36) strains were used.