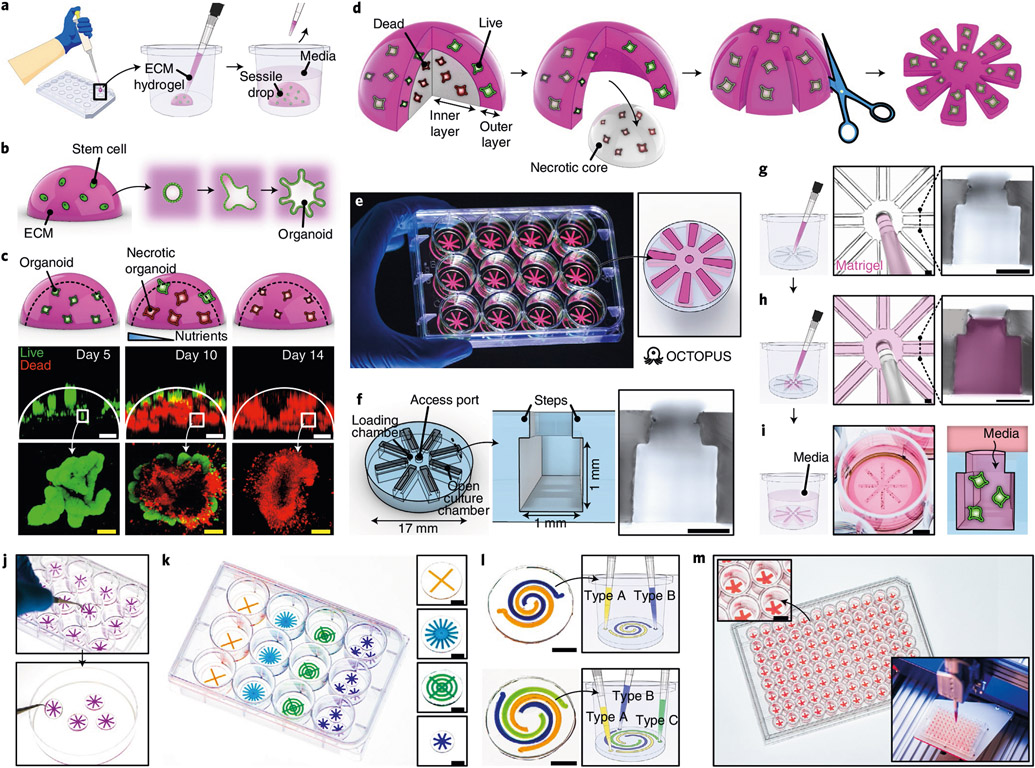
Fig. 1 ∣. Geometric engineering of conventional organoid culture using OCTOPUS.
a,b, Schematic showing conventional techniques that rely on 3D culture (a) and self-organization (b) of stem cells in sessile drops of ECM hydrogel to form organoids. c, Top row: schematic showing the formation of necrotic core in Matrigel drop culture. Middle and bottom rows: loss of viability and structural integrity in Matrigel drop culture of intestinal organoids over 10–14 d due to limited nutrient supply. Green and red show live and dead cells, respectively. Scale bars, 500 μm (top images) and 100 μm (bottom images). d, Conceptual diagram that shows the idea of reconfiguring the geometry of a conventional hydrogel drop scaffold to culture organoids without diffusion limitations. e, Photo of OCTOPUS inserts in a standard 12-well cell culture plate. f, Device design of OCTOPUS. The micrograph shows the cross-section of the culture chamber. Scale bar, 500 μm. g,h, Injection and distribution of stem-cell-containing Matrigel solution into the culture chambers of OCTOPUS. The micrographs show the top-down view of the access port with a 200-μl pipette tip (left) and the cross-section of the chamber (right) before (g) and after (h) gel injection. Pink shows Matrigel. Scale bars, 500 μm. i, OCTOPUS is kept submerged in media during culture. Developing organoids in the hydrogel are supplied with nutrients through the opening of the culture chambers. Scale bar, 5 mm. j, Facile handling and transferability of OCTOPUS. k, Examples of different chamber/device designs in OCTOPUS. Scale bars, 5 mm. l, Multi-chamber designs for co- and tri-culture in OCTOPUS. Scale bars, 5 mm. m, OCTOPUS can be deployed as a culture platform in a 96-well format coupled with a robotic fluid handling system to scale up the production of organoids. Scale bar, 3 mm.