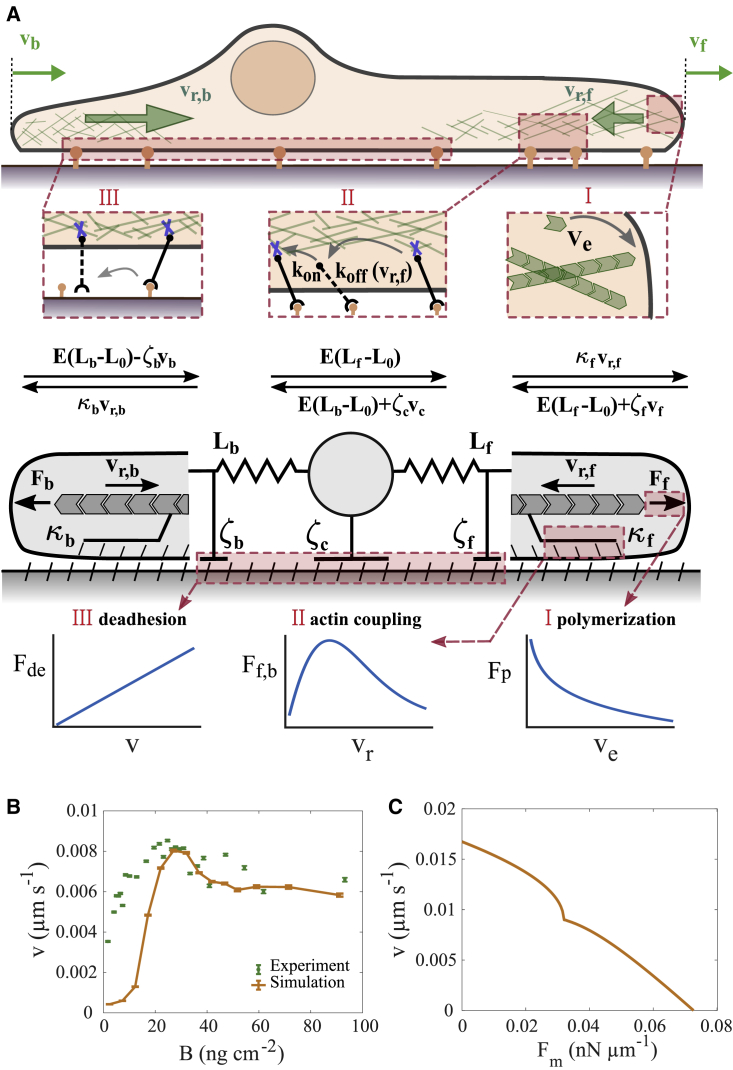
Figure 2.
(A) Model constituents. Cartoon of a cell moving on a 1D fibronectin lane (top), force balances (middle), and the mechanical components of the model (bottom). Front and back protrusion edges move with velocities and , respectively. The F-actin networks flow with the retrograde flow rates and , respectively. The forces and arise from polymerization of F-actin, act on the protrusion edge membrane, and drive retrograde flow against the friction forces. The front and back edge membrane experience drag with the coefficients and , respectively. Elastic forces and act between the cell body and the edges (equilibrium length ). The balance of the elastic forces determines the motion of the cell body against the drag force . Bottom panels illustrate types of essential relations of the model. The de-attachment force of the back is linearly related to velocity. The friction force between F-actin network retrograde flow and stationary structures exhibits a maximum in its dependency on retrograde flow (clutch). The polymerization force is logarithmically related to the network extension rate due to the force dependency of the polymerization rate. (B and C) Two constitutive relations. (B) The adhesion-velocity relation. Green dots represent experimental data (see Table S1 and Data S7). Error bars represent the standard error of the mean. (C) The stationary force-velocity relation predicted by the model for = 45 ng cm−2 (see section S10 for details). It provides the cell velocity under constant application of the external force to the leading edge or cell body. Parameter set 1 from Table S2 is used for both panels. To see this figure in color, go online.