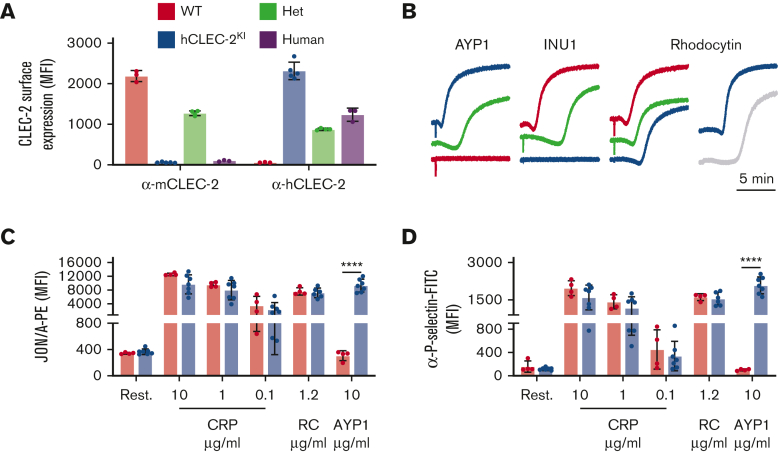
Figure 1.
hCLEC-2KImice have normal CLEC-2 expression and platelet activation. (A) Surface expression of mouse and human CLEC-2 by flow cytometry using INU1 and AYP1 antibodies, respectively. Heterozygous mice expressed half human and half mouse CLEC-2, whereas hCLEC-2KI mice expressed only hCLEC-2 on their platelet surface. (B) Light transmission aggregometry with washed platelets shows that AYP1 (10 μg/mL) and INU1 (10 μg/mL) cause aggregation of hCLEC-2KI and WT platelets, respectively, and reduced aggregation in hCLEC-2KI heterozygous platelets. RC-induced (0.24 μg/mL) aggregation has a longer lag time not only in hCLEC-2KI platelets but also in human platelets. (C) Platelet integrin activation measured by JON/A-PE antibody binding in flow cytometry shows no difference in (hem)ITAM-mediated platelet activation in hCLEC-2KI platelets and hCLEC-2–specific activation by AYP1. (D) Platelet granule secretion measured using an anti–P-selectin antibody in flow cytometry was unaltered in hCLEC-2KI following platelet activation by (hem)ITAM agonists, whereas AYP1 causes hCLEC-2–specific granule secretion. Data analyzed by two-way analysis of variance followed by a Sidak multiple comparison test. ∗∗∗∗P < .001. Results are shown as mean ± standard deviation, with each circle representing 1 individual and are representative of 3 independent experiments. CRP, collagen-related peptide; FITC, fluorescein isothiocyanate; Het, heterozygous; MFI, mean fluorescent intensity; PE, phycoerythrin; RC, rhodocytin.