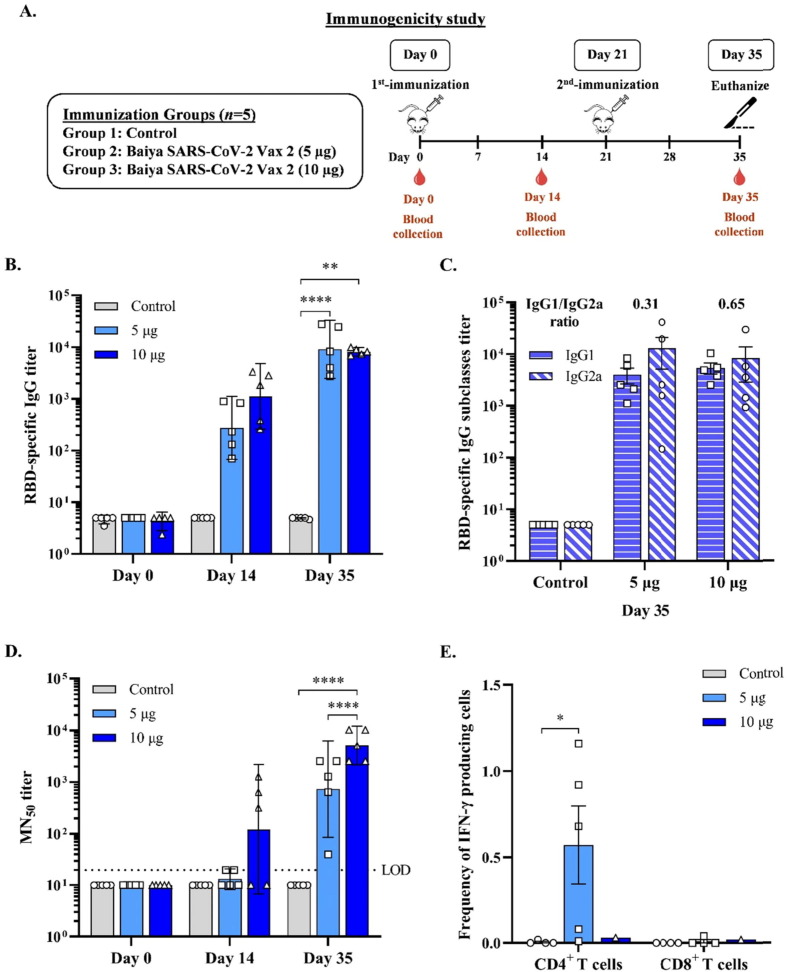
Fig. 1.
(A) Schematic representation showing the timeline of vaccine immunizations and blood collection in ICR mice. Mice were divided into 3 groups (n = 5): the control and Baiya SARS-CoV-2 Vax 2 (dose 5, 10 µg of RBD-Fc adjuvanted with 1 µg of 3 M-052-AF plus 50 µg Al content of alum) groups. The mice were intramuscularly injected twice on day 0 and 21, and blood was collected on day 0, 14, and 35,14 days after each immunization. (B) RBD-specific total IgG determined by ELISA (C) RBD-specific IgG subtype responses assessed by ELISA (D) 50 % neutralizing antibody (MN50) titer against SARS-CoV-2 clinical isolate as determined in a live virus microneutralization assay. Dotted lines indicate the assay limit of quantitation. The data are presented as geometric mean titers with 95 % confidence intervals. (E) Baiya SARS-CoV-2 Vax 2-specific T cell responses were measured by flow cytometry with intracellular cytokine straining of splenocytes harvested from mice immunized with the vaccine. Frequency of IFN-γ producing CD4+, and IFN-γ producing CD8+ T cells after stimulation with Wuhan-RBD peptide pool for 12 to 18 h. The data are presented as mean ± SEM. Two-way ANOVA, Tukey test, was used (*: p < 0.05, **: p < 0.01, ****: p < 0.0001). Values smaller than the limit of detection (LOD) are plotted as 0.5*LOD.