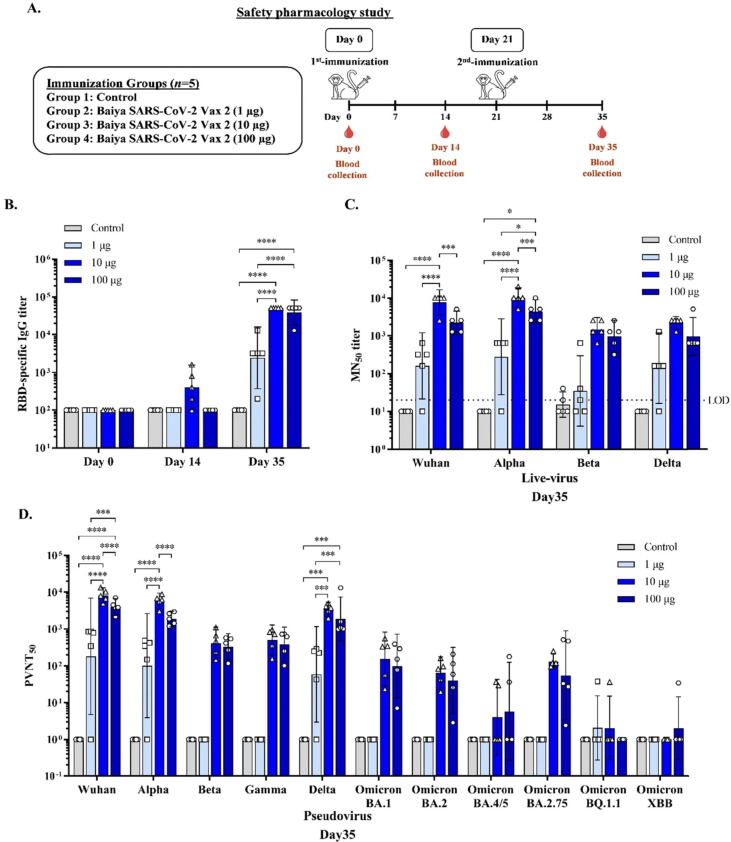
Fig. 4.
(A) Experimental design for the immunogenicity and safety pharmacology study in cynomolgus monkeys. Monkeys were divided into 4 groups (n = 5): the control and Baiya SARS-CoV-2 Vax 2 (dose 1, 10, and 100 µg of RBD-Fc adjuvanted with 5 µg of 3 M-052-AF plus 0.5 mg Al content of alum) groups. The monkeys were intramuscularly injected on Days 0 and 21, and blood was collected on day 0, 14, and 35 to assess the humoral immune response (B) RBD-specific total IgG (C) 50 % microneutralizing (MN50) titer, Values smaller than the limit of detection (LOD) are plotted as 0.5*LOD and (D) 50 % pseudovirus neutralizing titer (PVNT50) in the sera collected immunized monkeys were measured by ELISA. The data are presented as geometric mean titers with 95 % confidence intervals. The data are presented as mean ± SD. Two-way ANOVA, Tukey test, was used (*: p < 0.05, ***: p < 0.001, ****: p < 0.0001).