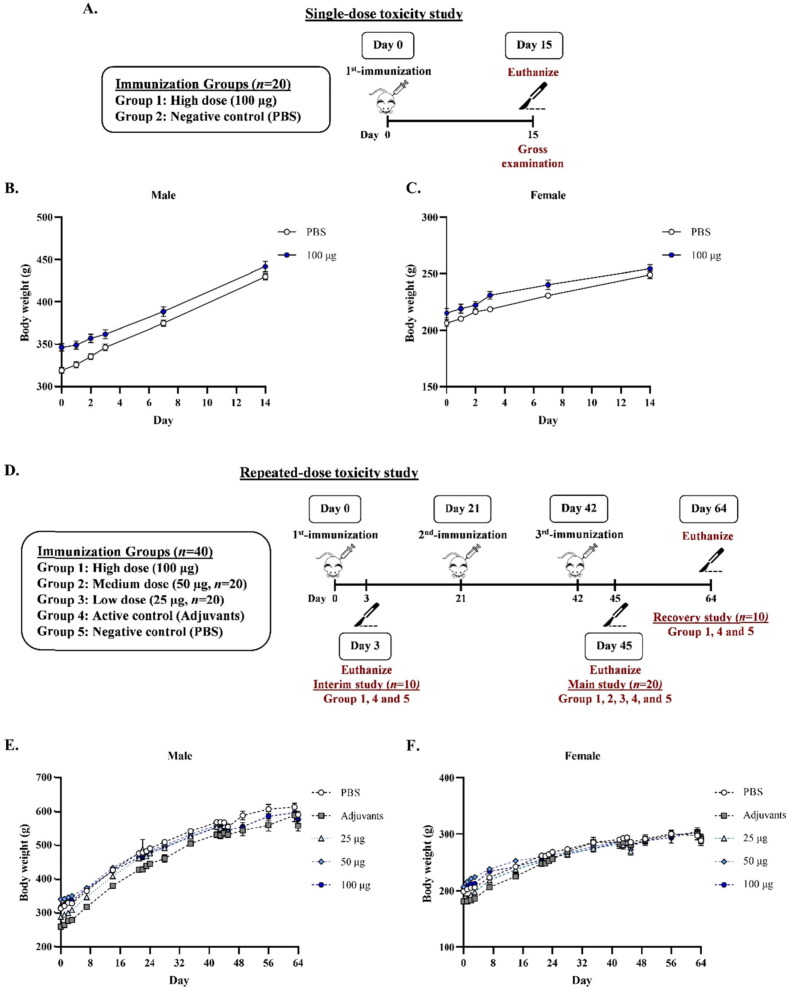
Fig. 5.
(A) Single-dose toxicity study design in Jcl:SD rats. Rats were divided into 2 groups (n = 10): the negative control (PBS) and high dose (100 µg) of Baiya SARS-CoV-2 Vax 2 groups. The rats were intramuscularly injected on day 0. On day 15, rats were euthanized and gross examined. The change in body weight of (B) male and (C) female rats was measured throughout the study. (D) Schematic representation of repeated-dose toxicity study in Jcl:SD rats. Rats were divided into 6 groups (n = 20–40), i.e., low, medium, and high doses (25, 50, and 100 µg) of Baiya SARS-CoV-2 Vax 2, active control (adjuvants), and negative control (PBS) groups. The rats were intramuscularly immunized on day 0, 21, and 42 (21 days interval). On day 3, 10 rats of high dose, active and negative controls groups were euthanized and gross examined as an interim study. After the third immunization on day 45, 20 rats of all groups were euthanized as a main study. Then, 22 days after the 3rd-immunization (day 64), the rest 10 rats of high dose, active and negative controls groups were euthanized as a recovery study. The change of body weight of (E) male and (F) female rats were measured throughout the study. The data are presented as mean ± SEM.