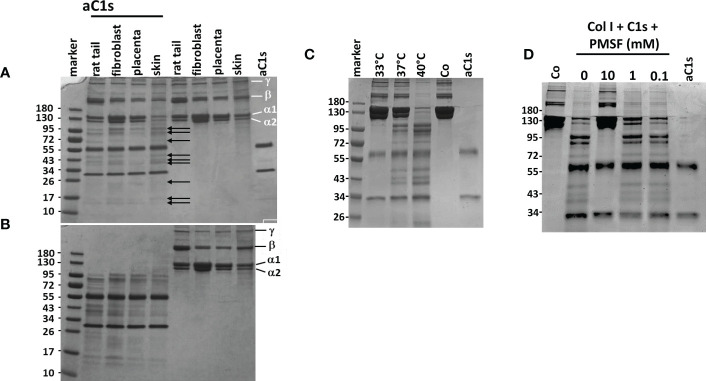
Figure 5.
Proteolytic activity of aC1s on procollagen I (A) Overnight incubation of collagen I from different sources with aC1s shows partial degradation into different smaller fragments as indicated by arrows. The degradation was independent from the source of collagen I Samples incubated without aC1s remained unaffected, as shown by the α1,α2, ß and γ collagen I chains. aC1s was represented by two bands, corresponding to the N-terminal chain at approx. 65 kDa and the C-terminal chain at approx. 32 kDa. (B) Denaturation of collagen I prior to incubation with aC1s shows almost complete degradation of procollagen I fibers into several smaller fragments. Samples incubated without aC1s remain unchanged. (C) C1s-mediated degradation of collagen I is temperature-dependent, with no degradation at an incubation temperature of 33°C, limited degradation at 37°C, and almost complete degradation at 40°C. These data suggest that the collagenase activity of aC1s depends on the unfolded state of collagen I (D) C1s collagenase activity is abolished by addition of the serine protease inhibitor PMSF at 10mM concentration, but lower PMSF concentrations have no inhibitory effect. The data are representative of five independent experiments.