Figure 3.
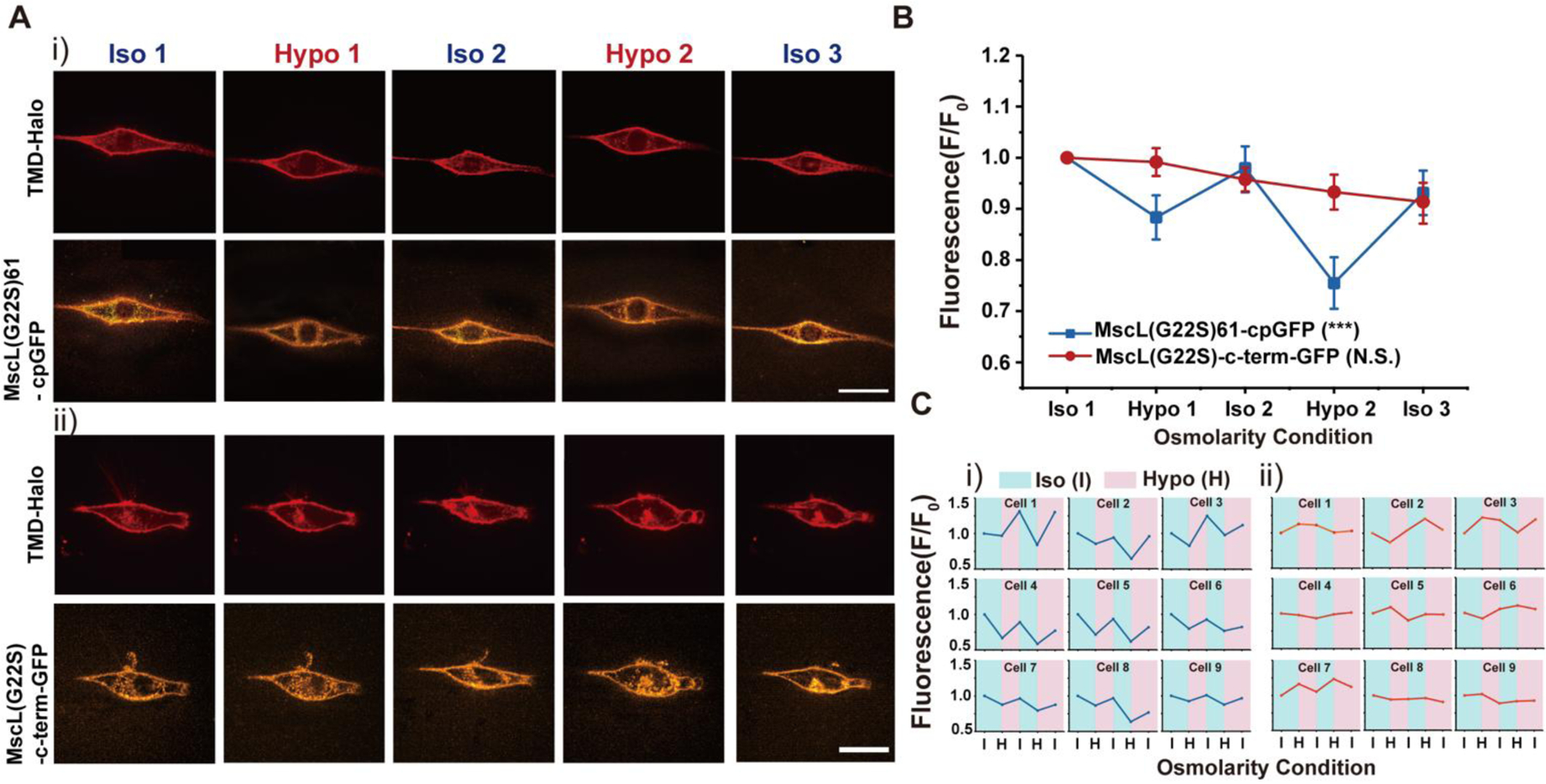
NIH3T3 cells transfected with the MscL tension reporter in response to cyclic pressure (A) (i) Confocal images showing the membrane localization of MscL tension reporter in NIH3T3 cells in response to the cyclic pressure test, which was carried out by alternating between iso-osmotic and hypo-osmotic conditions repeatedly. Transfected cells were cultured in iso-osmotic condition for 2 days. DI water was first added to the cell culture media to create hypo-osmotic environments and then 10X PBS solution was added back to the media to increase the osmolarity for iso-osmotic environments. The osmolarity of the media is ~330 mOsm for iso-osmotic conditions and ~165 mOsm for hypo-osmotic conditions. Each confocal image was taken four minutes after the addition of DI water or PBS solution. (ii) The confocal images showing the NIH3T3 cells transfected with the MscL(G22S)-c-term-GFP construct as a control in response to the same cyclic pressure cycle test as mentioned in (i). (B) Normalized fluorescence intensities of the cell membranes of NIH3T3 cells transfected with the MscL tension reporter and MscL(G22S)-c-term-GFP construct corresponding to the cyclic pressure cycle test. Nine cells were analyzed for each condition from three independent experiments. (C) (i) Fluorescence intensity traces of MscL(G22S)61-cpGFP on cell membranes of nine different NIH3T3 cells during the cyclic osmotic tests (ii) Fluorescence intensity traces of MscL(G22S)-c-term-GFP on cell membranes of nine different NIH3T3 cells as a control. Scale bars: 10 μm. The error bars denote standard error. ***: p < 0.001.
