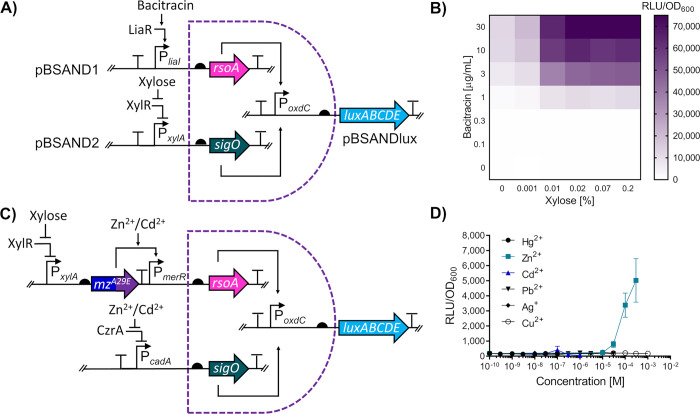
Figure 6.
Assessing the functionality and modularity of the two-subunit SigO-RsoA σ-factor two-input AND gate for B. subtilis. (A) Circuit schematic of the two-input AND gate. To test the functionality of the SigO-RsoA based AND gate, two promoters PliaI and PxylA, inducible by bacitracin and xylose, respectively, were used to induce the expression of both SigO and RsoA, allowing for transcription from the PoxdC promoter to drive luciferase (luxABCDE) expression. The sigO and rsoA genes are indicated via the teal and pink arrows, respectively. (B) Functionality of the SigO-RsoA AND gate using bacitracin and xylose. Cells with the integrated SANDBOX vectors incorporating a bacitracin and xylose inducible promoter were induced with various combinations of xylose and bacitracin with the heatmap used to show luciferase output across the tested concentrations. (C) Circuit schematic for an ultraspecific Zn2+ biosensor used the SigO-RsoA system. SigO and RsoA genes are indicated as previously described. The circuit, which utilizes the chimera MerRZntRA29E, is denoted via the split dark blue and purple arrow and simplified to “mzA29E”. For simplicity, the native B. subtilis metalloregulator CzrA present at a different genomic locus on the chromosome has not been indicated. (D) Dose response and specificity of the SigO-RsoA based Zn2+ detection circuit. For panels (B) and (D), cells were grown to OD600 = ∼0.03 and induced with the concentrations of either xylose and bacitracin (B) or metals (D) as indicated. Luciferase activity output (relative luminescence units [RLU]) was normalized to optical density (OD600) values (RLU/OD600) measured and averaged for three time points (35, 40, and 45 min) postinduction. Values are presented as mean ± standard deviation of two or three independent replicates.