Figure 1: ER-targeted reporters to investigate ribosome stalling at the ER.
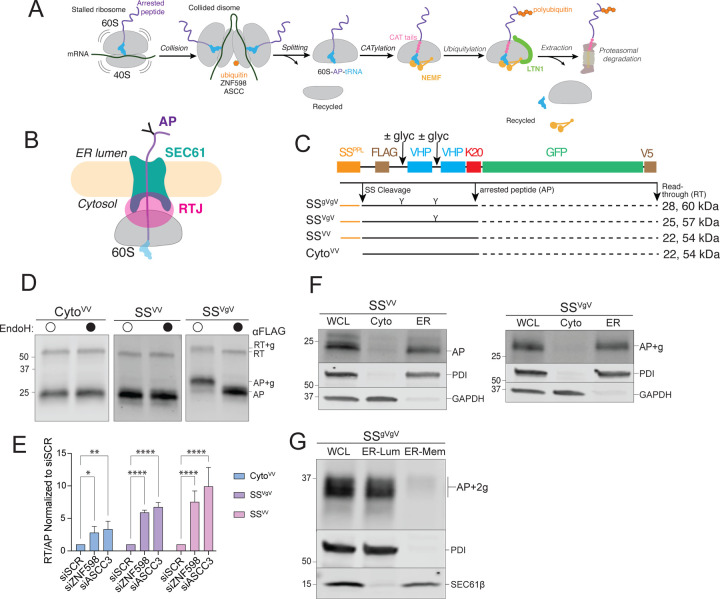
A: Schematic of ribosome-associated quality control (RQC) in the mammalian cytoplasm.
B: Topological organization of 60S-AP-tRNA stalled at an ER translocon. APs on ER ribosomes are topologically segregated from the cytosol by the ribosome-translocon junction (RTJ).
C: Schematic of the stalling reporters used in this study. SSPPL, signal sequence from bovine preprolactin; FLAG, FLAG epitope tag; ± glyc, presence or absence of an N-glycosylation sequon; “Y”, N-glycan; VHP, villin headpiece domain; K20; polylysine stalling sequence of 20 lysine residues; GFP, superfolder green fluorescent protein; V5, epitope tag. Composition of each reporter shown below, with predicted MW (indicated in kDa) for arrest peptide (AP, black line) or readthrough (RT, black line + dashed black line) species produced by each stalling reporter.
D: Endoglycosidase H (endoH) treatment on SSVgV reporter demonstrates glycosylation of SSVgV by increase in AP mobility. HEK293 cells were transfected with the indicated reporters and products were analyzed by immunoblot of whole cell lysates (WCLs) with FLAG antibody. Labels indicate mobilities of glycosylated (+g) and non-glycosylated RT and APs; data shown are representative of three independent experiments.
E: ER-stalled ribosomes are recognized and split by ZNF598 and ASCC3, respectively. HEK293 cells were transfected with scrambled (SCR), ZNF598, or ASCC3 small interfering RNAs (siRNA) and stalling reporter constructs CytoVV, SSVV, SSVgV. Quantification of RT and AP species was calculated as a ratio of RT/AP. Data are the mean ± SD of at least three independent experiments. *p < 0.05, **p < 0.01, ****p < 0.0001 determined by two-way ANOVA.
F: Cell fractionation analysis of subcellular AP distribution shows that ER-APs co-fractionate with ER markers. U2OS cells were transfected with the indicated reporters and subjected to cell fractionation. Reporter products were analyzed by immunoblot of WCL, cytosolic (Cyto), and ER cell fractions with FLAG antibody. GAPDH: cytosol marker; PDI: ER marker; data shown are representative of three independent experiments.
G: ER-APs predominantly localize in the ER lumen. HEK293 cells were transfected with SSgVgV. Cells were fractionated under conditions optimized to promote leakage of ER luminal contents while preserving membrane integrity as optimized in Fig S1E. Reporter products were analyzed by immunoblot of WCL, ER lumen (ER-Lum), and ER membrane (ER-Mem) fractions with FLAG antibody. PDI: ER lumen marker; SEC61β: ER membrane marker; data shown are representative of two independent experiments.