Figure 2: Proteasomal degradation of ER-AP requires dislocation to the cytosol via a p97/VCP-dependent and HRD1-independent pathway.
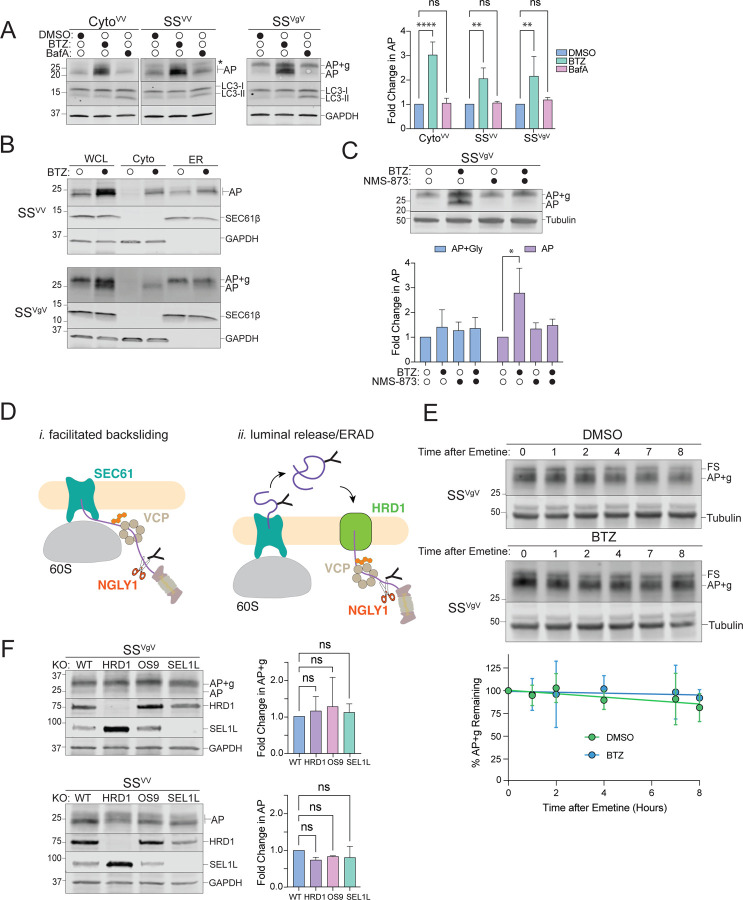
A: Cytosolic- and ER-APs are stabilized by proteasome but not lysosome inhibitors. Left panels, HEK293 cells expressing the indicated reporters were treated either with DMSO, 1 µM bortezomib (BTZ), or 100 nM Bafilomycin A1 (BafA) for 4 hr. WCLs were analyzed by immunoblot with FLAG antibody to detect AP and AP+g species, and LC3 antibody to assess the effect of BafA treatment on LC3-II accumulation. Asterisks indicate a nonspecific immunoreactive band. GAPDH: loading control. Right panel, Quantification of immunoblot data as indicated. Fold change in AP relative to DMSO treated cells was calculated after normalization to GAPDH. Data are the mean ± SD of at least three independent experiments. **p < 0.01, ****p < 0.0001 determined by two-way ANOVA.
B: ER-APs are dislocated to the cytosol prior to degradation via the proteasome. U2OS cells were transfected with the indicated reporter and treated with DMSO or 1 µM BTZ for 4 hr prior to cell fractionation. Reporter products were analyzed by immunoblot of WCL, Cyto, and ER cell fractions with FLAG antibody. GAPDH: cytosol marker; SEC61β: ER marker; data shown are representative of three independent experiments.
C: ER-AP dislocation to the cytosol depends on p97/VCP. Upper panel, HEK293 cells expressing SSVgV were treated with 1 µM BTZ, 5 µM NMS-873, or 1 µM BTZ and 5 µM NMS-873 for 4 hr. Reporter products were analyzed by immunoblot with FLAG antibody. Tubulin: loading control. Lower panel, Quantification of immunoblot data as indicated. Data are the mean ± SD of two independent experiments. *p < 0.05 determined by two-way ANOVA.
D: Two models of ER-AP dislocation to the cytosol via p97/VCP and degradation by the proteasome. Details in text.
E: Turnover of glycosylated SSVgV is slow. Upper panels, HEK293 cells transfected with SSVgV (“Original” reporter, see figure S2F) were treated with 20 µM emetine and either DMSO or 1 µM BTZ for the indicated times. Reporter products were analyzed by immunoblot with FLAG antibody. Tubulin: loading control. Lower panel, Quantification of immunoblot data as indicated. %AP+g remaining was determined by normalizing AP+g signal to tubulin signal, then calculating the fraction remaining relative to time = 0 hr. Data are the mean ± SD of two independent experiments.
F: ER-AP degradation does not require the HRD1 retrotranslocon. Left panels, HEK293 WT, HRD1KO, OS9KO, and SEL1LKO cell lines were transfected with the indicated reporters. Reporter products were analyzed by immunoblot with FLAG antibody. Knockouts were confirmed by blotting with antibodies against endogenous HRD1 or SEL1L proteins. GAPDH: loading control. Right panels, Quantification of AP intensity for SSVV and AP+g intensity for SSVgV. Fold changes relative to WT cells were calculated after normalization to GAPDH. Data are the mean ± SD of at least two independent experiments. ns > 0.05, determined by one-way ANOVA.
F: Schematic of the two reporter variants illustrating the frameshift (FS) species generated by our stalling reporters as described in the materials and methods section. Original: frameshift product generated by this reporter is ~25kD. FS-corrected: frameshift product generated by this reporter is 60kD or 65kD. S Tag+1 and S Tag+2 are generated by out of frame translation downstream of the stalling sequence (K20). The Original reporter was used in Figures 2E, S2A. The FS-corrected reporter is used in all other experiments.