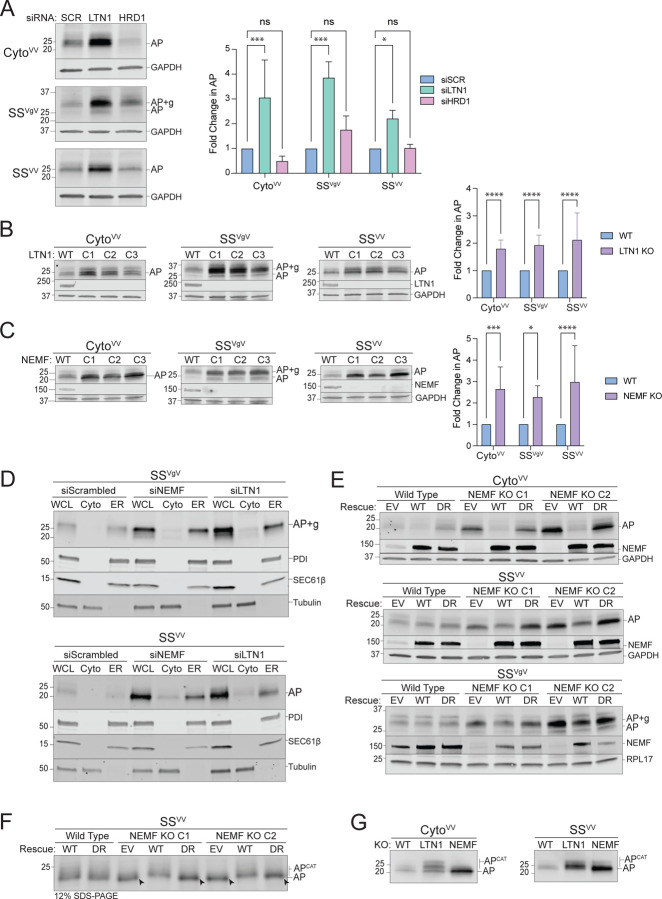
Figure 3: ER-AP degradation requires RQC machinery.
A: Knockdown of LTN1 but not HRD1 stabilizes both cytosolic- and ER-APs. Left panels, HEK293 cells were transfected with the indicated siRNAs and the indicated stalling reporters. Reporter products were analyzed by immunoblot with FLAG antibody. GAPDH: loading control. Right panel, Quantification of AP intensity for CytoVV and SSVV and AP+g intensity for SSVgV. Fold change relative to WT cells was calculated after normalization to GAPDH. Data are the mean ± SD of at least 3 independent experiments. *p < 0.05, ***p < 0.001, determined by two-way ANOVA.
B: LTN1 is required for degradation of cytosolic- and ER-APs. Left panels, HEK293 WT and clonal LTN1KO cell lines (C1, C2, C3) were transfected with the indicated reporters. Reporter products were analyzed by immunoblot with FLAG antibody. Knockouts were confirmed by blotting with antibodies against endogenous LTN1 protein. GAPDH: loading control. Right panel, Quantification as in Fig 3A. Fold change relative to WT cells was calculated after normalization to GAPDH. Data are the mean ± SD of at least 3 independent experiments. ****p < 0.0001, determined by two-way ANOVA.
C: NEMF is required for degradation of cytosolic- and ER-APs. Left panels, HEK293 WT and clonal NEMFKO cell lines (C1, C2, C3) were transfected with the indicated reporters. Reporter products were analyzed by immunoblot with FLAG antibody. Knockouts were confirmed by blotting with antibodies against endogenous NEMF protein. GAPDH: loading control. Right panel, Quantification as in Fig 3A. Fold change relative to WT cells was calculated after normalization to GAPDH. Data are the mean ± SD of at least 3 independent experiments. *p < 0.05, ***p < 0.001, ****p < 0.0001, determined by two-way ANOVA.
D: Disruption of RQC favors luminal release of ER-APs. U2OS cells were transfected with indicated siRNAs and stalling reporters followed by cell fractionation. Reporter products were analyzed by immunoblot of WCL, Cyto, and ER cell fractions with FLAG antibody. Tubulin: cytosol marker; SEC61β and PDI: ER markers.
E: CATylation is required for ER-AP degradation. HEK293 WT or clonal NEMFKO cells (C1 and C2) were transfected with empty vector (EV), WT NEMF, or NEMF-DR and the indicated stalling reporters. Reporter products were analyzed by immunoblot with FLAG antibody. Endogenous and ectopic NEMF expression validated by immunoblotting with anti-NEMF antibody. GAPDH or RPL17: loading controls.
F: ER-APs are CATylated. HEK293 WT or clonal NEMFKO cells (C1 and C2) were transfected with EV, WT, or DR NEMF and the indicated stalling reporters as in panel E. SSVV-transfected cell lysates were separated by 12% SDS-PAGE and analyzed by immunoblot with FLAG antibody. Unmodified APs are indicated by the label “AP” and by arrowheads; CATylated APs are indicated by “APCAT”; data shown are representative of two independent experiments.
G: CATylated ER-APs accumulate in LTN1-deficient cells. HEK293 cells were transfected with the indicated siRNAs and stalling reporters. Reporter products were analyzed by immunoblot with FLAG antibody. AP and APCAT labels indicate unmodified and CATylated APs, respectively; data shown are representative of two independent experiments.