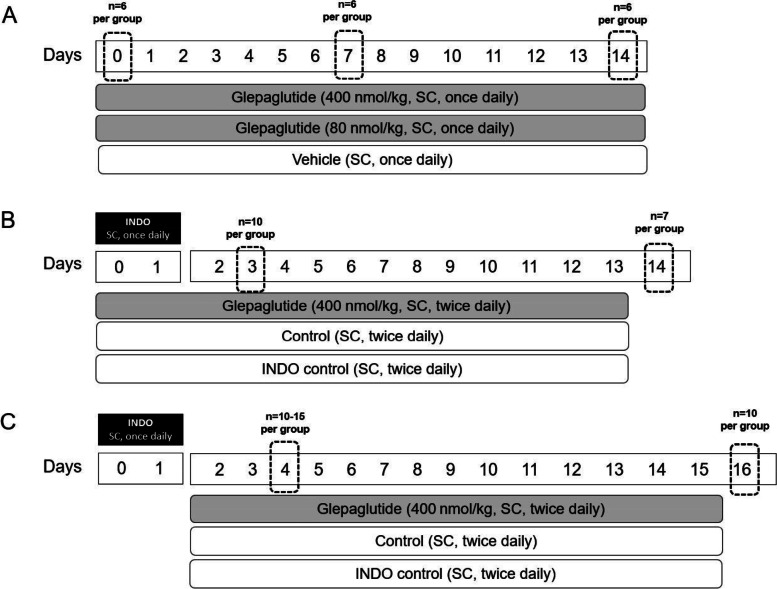
Fig. 1.
Study design diagram for the assessment of the intestinotrophic effect of glepaglutide in naive rats and its effects in the INDO-induced small intestinal inflammation model in a co-treatment and post-treatment regimen. A Naive Wistar rats were treated with glepaglutide 400 nmol/kg, glepaglutide 80 nmol/kg, or vehicle (SC, twice daily) for 14 days. B/C Naive Wistar rats were treated with INDO 7 mg/kg (SC, once daily) for 2 consecutive days. Rats with small intestinal inflammation were treated with glepaglutide 400 nmol/kg, control, or INDO control (SC, twice daily) in B co-treatment for 14 days or C post-treatment for 16 days. The dashed rectangles show sacrifice dates. INDO, indomethacin; SC, subcutaneously