Abstract
Aims
To perform a comparative analysis of right ventricle (RV) myocardial mechanics, assessed by 2D speckle-tracking echocardiography (2D-STE), between patients with Fabry disease and patients with sarcomeric disease.
Methods and results
Patients with Fabry cardiomyopathy (FC) (n = 28) were compared with patients with sarcomeric hypertrophic cardiomyopathy (HCM), matched for degree of left ventricle hypertrophy (LVH) and demographic characteristics (n = 112). In addition, patients with Fabry disease and no LVH [phenotype-negative carriers of pathogenic α-galactosidase gene mutations (GLA LVH-)] (n = 28) were compared with age and sex-matched carriers of sarcomeric gene mutations without LVH [Phenotype-negative carriers of pathogenic sarcomeric gene mutations (Sarc LVH-)] (n = 56). Standard echocardiography and 2D-STE were performed in all participants. Despite a subtle impairment of RV global longitudinal strain (RV-GLS) was common in both groups, patients with FC showed a more prominent reduction of RV free wall longitudinal strain (RV-FWS) and lower values of difference between RV-FWS and RV-GLS (ΔRV strain), in comparison to individuals with HCM (P < 0.001 and P = 0.002, respectively). RV-FWS and ΔRV strain demonstrated an independent and additive value in discriminating FC from HCM, over the presence of symmetric LVH, systolic anterior motion of the mitral valve and RV hypertrophy. Similar results were found in GLA LVH- patients: they had worse RV-FWS and lower values of ΔRV strain as compared to Sarc LVH- patients (both P < 0.001).
Conclusion
Patients with FC show a specific pattern of RV myocardial mechanics, characterized by a larger impairment of RV-FWS and lower ΔRV strain in comparison to patients with HCM, which may be helpful in the differential diagnosis between these two diseases.
Keywords: Fabry disease, hypertrophic cardiomyopathy, strain analysis, speckle-tracking echocardiography, right ventricle
Graphical Abstract
Graphical Abstract.
Introduction
Fabry disease is a rare lysosomal storage disorder, often misdiagnosed and with poor outcome if left untreated. Cardiac involvement is frequent and represents the strongest determinant of prognosis.1 Left ventricular hypertrophy (LVH) is the hallmark of Fabry cardiomyopathy (FC), which may be indistinguishable from sarcomeric hypertrophic cardiomyopathy (HCM) using conventional echocardiography.1
Right ventricular hypertrophy (RVH) is found in a consistent proportion of patients with FC,2–4 while is less frequent and usually milder in HCM.5–7 In both diseases, right ventricular (RV) systolic function is typically normal when assessed by conventional echocardiography.2–8 Two-dimensional speckle-tracking echocardiography (2D-STE) allows to detect subtle RV impairment in different clinical settings, including FC9,10 and HCM.7,11,12 However, potential differences between FC and HCM in terms of RV strain analysis have never been investigated. The aim of the current study was to perform a comparative analysis of RV myocardial mechanics assessed by 2D-STE in: (i) patients with sarcomeric HCM vs. patients with FC and (ii) phenotype-negative carriers of pathogenic sarcomeric gene mutations (Sarc LVH-) vs. phenotype-negative carriers of pathogenic α-galactosidase (GLA) gene mutations (GLA LVH-).
Methods
Study population
We retrospectively screened two cohorts of: (i) patients with Fabry disease (n = 74), evaluated at the Fondazione Policlinico A. Gemelli IRCCS, Rome, Italy between January 2010 and January 2021 and (ii) patients with pathogenic sarcomeric mutations (n = 293), evaluated at the Leiden University Medical Center (LUMC), Leiden, The Netherland during the same period.
As currently recommended,1 the diagnosis of Fabry disease was based on the measurement of the enzymatic activity of α-galactosidase A in leucocytes (for male patients) and confirmed by genetic testing demonstrating pathogenetic variants in the GLA gene (for all patients). In the second study cohort, HCM-causing mutations were identified by direct DNA sequencing of sarcomeric genes, performed through clinical evaluation (patients with LVH or family history of HCM). Genotypic information for both study populations are reported in Supplementary data online, Table S1.
Exclusion criteria from the present study were: age <18 years (n = 14), obstructive form (defined as maximum resting gradient >30 mmHg and/or maximum provocable gradient >50 mmHg, n = 33), insufficient quality of echocardiographic images (n = 38), known coronary artery disease (n = 8).
Both populations were divided in two groups according to left ventricular (LV) wall thickness: patients with LVH (defined as LV thickness ≥13 mm) were considered as those with an overt cardiac phenotype (FC and HCM). Patients without LVH (LV wall thickness <13 mm) were regarded as genetic positive but phenotype negative (GLA LVH- and Sarc LVH-).13
For the purpose of the present study, FC patients were compared with HCM patients matched for maximal LV wall thickness and demographic characteristics in a 1:4 ratio. In addition, GLA LVH- patients were compared with age and sex-matched Sarc LVH- individuals in a 1:2 ratio.
The Fabry population was previously enrolled in ongoing clinical and echocardiographic registries (Fabry Outcome Survey, Fabry Registry and Follow Me Registry) and all patients signed a dedicated informed consent. Due to the retrospective design of this study, the Medical Ethical Committee of the LUMC declared that no formal ethical approval was needed and waived the need for written informed consent. The study was conducted in accordance with the principles of the Helsinki Declaration.
Conventional echocardiographic assessment
Comprehensive echocardiographic examinations were performed with the patients at rest in the left lateral decubitus position using commercially available ultrasound systems (Toshiba ArtidaTM, Toshiba Medical System, Tokio, Japan or Philips EPIQ CVx, Philips Medical Systems, Andover, Massachusetts, USA in the Policlinico A. Gemelli; E7, E9 and E95 system, General Electric Vingmed, Horten, Norway or Philips EPIQ 7, Philips Medical Systems, Andover, Massachusetts, USA in the LUMC). Two-dimensional, colour, pulsed-wave, and continuous-wave Doppler images were acquired from the parasternal, apical and subcostal views. All images were digitally stored for offline analyses, which were performed by an experienced operator (M.C.M.), in both study centres.
From a short-axis view at basal, mid, and apical levels the maximal LV end-diastolic wall thickness was assessed. LV linear dimensions were measured from parasternal long-axis views, and LV mass was calculated according to the Devereux formula and indexed for body surface area.14 The asymmetry of LVH was quantified using the ratio between the thickness of the interventricular septum and the posterior wall (IVS-PW thickness ratio). Chamber quantification, LV ejection fraction (LVEF) and diastolic function were assessed according to the most recent guidelines.14,15
A comprehensive assessment of RV geometry, size and systolic function was performed according to current recommendations.16 RV free wall thickness was derived from 2D echocardiography using the subcostal view; RV hypertrophy was defined as RV wall thickness >5 mm. RV end-diastolic and end-systolic area were measured from a RV focused apical four-chamber view. RV systolic function was evaluated using tricuspid annular plane systolic excursion (TAPSE) by M-mode, tricuspid annular peak systolic velocity (RV S′ velocity) by pulsed-wave tissue Doppler imaging (TDI) and RV fractional area change (RVFAC). TAPSE <17 mm, RV S′ velocity <9.5 cm/s and RVFAC <35% were considered suggestive of RV systolic dysfunction.16
The presence of systolic anterior movement (SAM) of the mitral valve apparatus was visually assessed from the parasternal and apical long-axis views. The peak velocity of the tricuspid regurgitant jet was derived using continuous-wave Doppler, from a RV focused apical four-chamber view.16 Finally, the severity of mitral regurgitation and tricuspid regurgitation was evaluated using a multiparametric approach, as recommended.
Two-dimensional speckle-tracking echocardiographic analysis
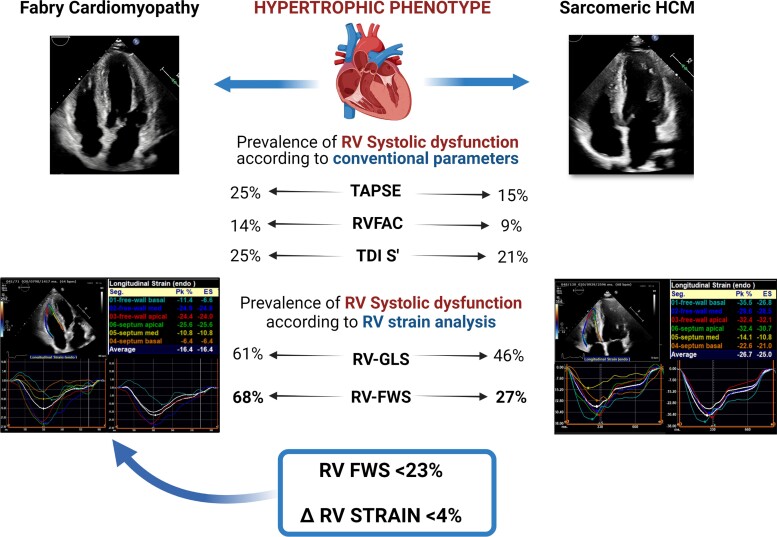
2D-STE analysis was performed offline using commercially available, vendor-independent, dedicated software, 2D Cardiac Performance Analysis© by TomTec-Arena TM (TomTec Imaging Systems, Unterschleissheim, Germany). Images from the apical 4- and 2-chamber and long-axis views were used for the assessment of LV global longitudinal strain (LV-GLS), while the RV focused apical four-chamber view was used to obtain RV global longitudinal strain (RV-GLS) and RV free wall longitudinal strain (RV-FWS).14,17 Images with frame rates between 50 and 90 were selected for 2D-STE analysis. The endocardial border was traced from an end-systolic frame by using a point-and-click approach. The region of interest was defined by the software and manually adapted to include the entire myocardial thickness. Then, the myocardium was automatically divided in six segments in each view. For the assessment of RV strain, the average values of the longitudinal peak systolic strain from the three segments of the free wall (RV-FWS) and from all six segments of the free wall and septal wall of the RV (RV-GLS) were calculated.17,18 For the assessment of LV-GLS, the average of the longitudinal peak systolic values obtained from the 17 LV segments in the three apical views was evaluated.14 In the present study, the values of strain measurements are reported in absolute values. According to current evidence,18 RV-GLS <20% and RV-FWS <23% were considered as impaired RV systolic function. In addition, the difference between RV-FWS and RV-GLS, referred to as ΔRV strain, was investigated as marker of the equilibrium of RV mechanical properties. RV strain is typically higher in the free wall as compared to the septum and a reference range of 5 ± 2% for ΔRV strain has been reported in healthy subjects.18
Statistical analysis
Normally-distributed continuous variables are presented as mean ± standard deviation whereas non-normally distributed data are presented as median and interquartile range. Categorical variables are expressed as frequencies and percentages. Comparison of clinical and echocardiographic characteristics between groups was performed by the unpaired Student’s t-test (for normally-distributed continuous variables), Mann–Whitney U test (for non-normally distributed continuous variables) and χ2 test or the Fisher’s exact test, as appropriate (for categorical variables). Multiple comparisons of continuous variables were tested with Bonferroni correction. The correlation between RV-FWS and RV free wall thickness was assessed using the Spearman’s method. Univariable and multivariable binary logistic regression analyses were performed to investigate the association between echocardiographic characteristics and FC (vs. HCM). Variables with a significant correlation at univariable analysis (P < 0.05) were included in the multivariable analysis. The odds ratio (OR) and 95% confidence intervals (CIs) were calculated for each variable. The goodness of fit of the univariable and multivariable regression models was evaluated by calculating Harrell’s C statistic with 95% CI. Since ΔRV strain has not been previously investigated in the context of cardiomyopathies with hypertrophic phenotype, a penalized spline curve was fitted to specifically characterize the association between FC and ΔRV strain. The spline curve displayed the changes of FC probability (expressed as percentage) across a range of ΔRV strain values and, based on this analysis, a threshold value of ΔRV strain to predict FC was proposed. Additionally, receiver-operating characteristics (ROC) curve analyses were performed to determine the optimal cut-offs of RV-FWS and ΔRV strain for discriminating FC from HCM. Different measures of diagnostic accuracy were computed for the relevant thresholds of RV-FWS and ΔRV strain.
Twenty random individuals (10 from each study cohort) were selected for the evaluation of intra- and inter-observer variability of RV strain parameters. Excellent agreement was defined by an intraclass correlation coefficient > 0.9, whereas good agreement was defined by a value between 0.75 and 0.90.
All tests were two-sided and P-values < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS version 25.0 (IBM Corporation, Armonk, NY, USA) and R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Phenotype-positive patients
Clinical and echocardiographic characteristics of FC and HCM patients are summarized in Table 1. A total of 140 patients with LVH (28 FC and 112 HCM) were included.
Table 1.
Clinical and echocardiographic characteristics of the phenotype-positive population
| Overall LVH+ (N = 140) | HCM (N = 112) | FC (N = 28) | P-value* | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| ȃAge (years) | 55 (42–64) | 54 (41–63) | 55 (51–65) | 0.128 |
| ȃFemale (n,%) | 30 (21) | 23 (21) | 7 (25) | 0.607 |
| ȃNYHA class (n,%) | 0.240 | |||
| ȃȃI/II | 128 (91) | 104 (93) | 24 (86) | |
| ȃȃIII/IV | 12 (9) | 8 (7) | 4 (14) | |
| ȃHypertension (n,%) | 49 (35) | 39 (35) | 10 (36) | 0.929 |
| ȃCOPD (n,%) | 7 (5) | 6 (5) | 1 (4) | 0.739 |
| ȃDiabetes (n,%) | 3 (3) | 3 (3) | 0 (0) | 1.000 |
| ȃPrevious history of AF (n,%) | 31 (22) | 29 (26) | 2 (7) | 0.033 |
| Echocardiographic characteristics a | ||||
| ȃMaximum LVWT (cm) | 2.0 (1.6–2.3) | 2.0 (1.7–2.4) | 1.8 (1.5–2.2) | 0.090 |
| ȃLV mass index (g/m2) | 151 (132–191) | 149 (132–182) | 167 (132–228) | 0.106 |
| ȃIVS/PW ratio | 1.4 (1.1–1.6) | 1.5 (1.2–1.6) | 1.1 (1.0–1.3) | <0.001 |
| ȃLVEDV index (ml/m2) | 51 (43–60) | 51 (43–60) | 50 (42–60) | 0.698 |
| ȃLVEF (%) | 62 ± 8 | 62 ± 8 | 62 ± 4 | 0.843 |
| ȃLV-GLS (%) | 16.3 (13.1–18.1) | 16.3 (13.2–18.2) | 15.6 (10.5–17.0) | 0.153 |
| ȃLAV index (ml/m2) | 41 (32–54) | 40 (30–51) | 48 (34–60) | 0.069 |
| ȃE/A ratio | 1.1 (0.8–1.5) | 1.1 (0.8–1.5) | 1.1 (0.8–1.3) | 0.481 |
| ȃE/E′ ratio | 10.3 (8.1–13.3) | 10.4 (7.9–13.4) | 10.4 (8.7–13.0) | 0.660 |
| ȃMitral SAM | 43 (31) | 40 (36) | 3 (11) | 0.010 |
| ȃMR grade (n,%) | 0.383 | |||
| ȃȃTrivial/Mild | 131 (94) | 106 (95) | 25 (89) | |
| ȃȃModerate | 9 (6) | 6 (5) | 3 (11) | |
| ȃRVWT (mm) | 5.5 (4.2–7.0) | 5.0 (4.0–6.5) | 7.0 (5.5–8.5) | <0.001 |
| ȃRVEDA (cm2) | 18.3 (15.3–21.0) | 18.3 (15.5–21.2) | 17.6 (14.8–20.5) | 0.453 |
| ȃRVESA (cm2) | 9.5 (8.0–11.3) | 9.5 (8.0–11.4) | 9.3 (7.9–11.5) | 0.976 |
| ȃRAV index (ml/m2) | 24 (16–29) | 22 (16–29) | 25 (18–30) | 0.185 |
| ȃTAPSE (mm) | 21 ± 4 | 21 ± 4 | 20 ± 3 | 0.085 |
| ȃRVFAC (%) | 46 ± 8 | 46 ± 8 | 46 ± 9 | 0.772 |
| ȃRV S′ velocity (cm/s) | 11.3 ± 2.8 | 11.4 ± 2.6 | 11.1 ± 3.1 | 0.566 |
| ȃRV-GLS (%) | 20.6 (16.5–23.6) | 21.2 (17.5–23.8) | 17.9 (14.2–21.8) | 0.010 |
| ȃRV-FWS (%) | 25.6 (20.7–28.1) | 26.0 (22.6–28.4) | 21.4 (17.1–23.9) | <0.001 |
| ȃΔRV strain (%) | 4.5 (2.8–6.0) | 4.8 (3.0–6.5) | 3.1 (1.7–4.8) | 0.002 |
| ȃTR jet velocity (m/s) | 2.4 (2.1–2.6) | 2.4 (2.1–2.6) | 2.4 (2.2–2.7) | 0.615 |
| ȃTR grade (n,%) | 0.584 | |||
| ȃȃTrivial/mild | 136 (97) | 108 (96) | 28 (100) | |
| ȃȃModerate | 4 (3) | 4 (4) | 0 (0) | |
All patients had sinus rhythm during the echocardiographic examination.
Comparison of echocardiographic parameters was performed applying a Bonferroni correction. P values <0.0028 were considered statistically significant (18 comparisons) and are shown in bold type.
AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; IVS/PW, interventricular septum/posterior wall; LAV, left atrial volume; LVEDV, LV end diastolic volume; LVWT, LV wall thickness; MR, mitral regurgitation; RAV, right atrial volume; RVEDA, RV end-diastolic area; RVESA, RV end-systolic area; RVWT, RV wall thickness; TR, tricuspid regurgitation.
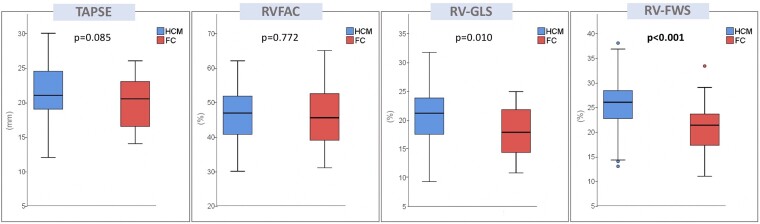
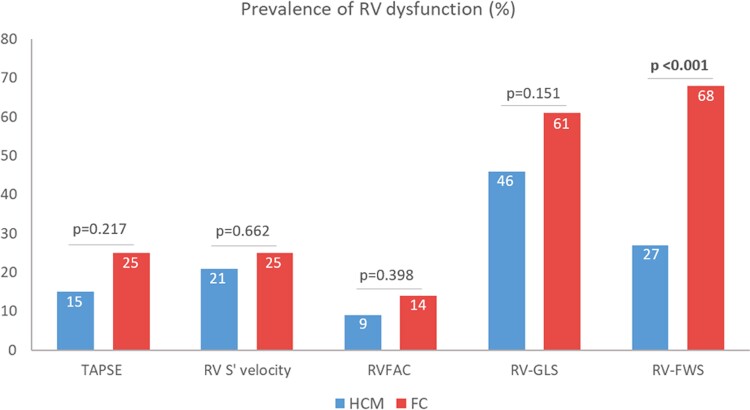
FC and HCM patients were similar for most of the echocardiographic parameters, such as LV and RV dimensions, LVEF, LV-GLS as well as parameters of LV diastolic function. In accordance with the selection criteria, the degree of LVH was similar between the two groups, but FC patients showed a more symmetric pattern of LVH, as expressed by a significantly lower IVS-PW thickness ratio (P < 0.001). Mitral SAM (without LV outflow tract gradient) was more frequently observed in HCM patients than FC patients (P = 0.010). FC patients had significantly increased RV wall thickness as compared to HCM patients (P < 0.001). Accordingly, the prevalence of RVH was higher in the FC group (93% vs. 44%, P < 0.001). The assessment of RV systolic function using conventional echocardiographic parameters did not show significant differences between the two groups and TAPSE, RVFAC and RV S′ were within the normal range in most of the patients. On the contrary, RV strain analysis revealed a subclinical RV systolic impairment in a sizeable proportion of subjects in both groups. Specifically, RV-GLS was impaired (<20%) in 61% of FC patients and 46% of HCM patients, with no significant difference between the two populations (P = 0.151). Conversely, abnormal RV-FWS (<23%) was more common in FC individuals than HCM patients (68% vs. 27%, P < 0.001), with significantly lower values of ΔRV strain in FC vs. HCM (P = 0.002). Figure 1 shows the comparison of RV parameters in FC vs. HCM. Figure 2 illustrates the proportion of patients with RV systolic dysfunction based on different echocardiographic parameters. Notably, there were no significant differences in RV strain parameters between patients with disease-causing mutations of the MYBPC3 gene (72% of the HCM patients) and patients with other sarcomeric gene mutations (see Supplementary data online, Table S2). In the overall population, RV-FWS was moderately correlated with RV wall thickness (correlation coefficient = 0.370; P < 0.001).
Figure 1.
Box plots showing echocardiographic parameters of RV systolic function in phenotype-positive patients. Lower, middle, and upper hinges of the box correspond to the 25th, 50th, and 75th percentiles. The upper and lower whiskers extend from the hinge to the largest and smallest value, respectively, no further than 1.5 times the interquartile range of the hinge.
Figure 2.
Prevalence of RV systolic dysfunction according to different echocardiographic parameters in phenotype-positive patients. The following thresholds were used to define RV systolic dysfunction: TAPSE <17 cm; RV S′ velocity <9.5 m/s; RVFAC < 35%; RV-GLS <20%; RV-FWS <23%.
Diagnostic value of RV mechanics to discriminate FC vs. HCM
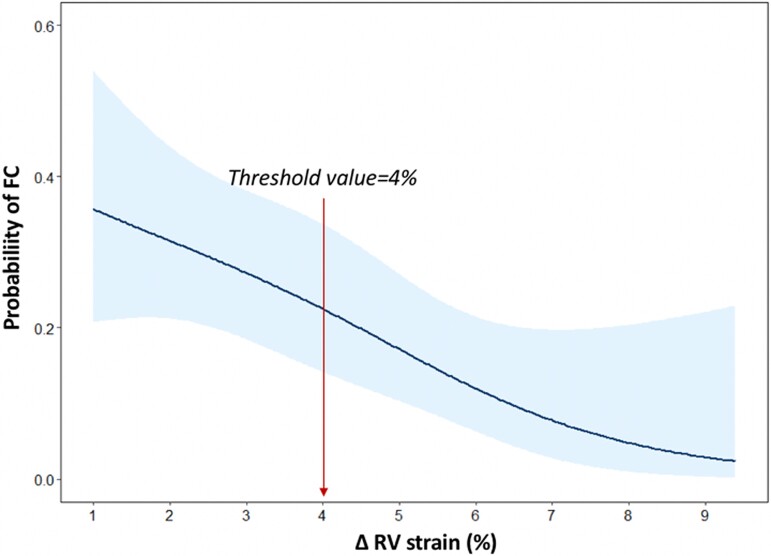
At univariate logistic regression analysis, a significant correlation was found between FC and the following echocardiographic variables: IVS-PW thickness ratio, mitral SAM, RV wall thickness, RV-GLS, RV-FWS and ΔRV strain (see Supplementary data online, Table S3). For collinearity reasons, different multivariable logistic regression models including IVS-PW thickness ratio, mitral SAM, RV wall thickness and one parameter of RV mechanics were built (Table 2). After correcting for IVS-PW thickness ratio, mitral SAM and RV wall thickness, RV-GLS was not significantly associated with FC (P = 0.141). Conversely, both RV-FWS and ΔRV strain retained an independent association with FC (P = 0.005 and P = 0.002, respectively). Notably, the likelihood ratio test demonstrated a significant improvement in the predictive value with the addition of RV-FWS or ΔRV strain to the baseline model (P = 0.003 and P = 0.005, respectively). The multivariate model incorporating ΔRV strain yielded the highest increment in the C statistic (0.881, 95% CI: 0.809–0.953). Therefore, a spline curve was specifically performed to characterize the association between ΔRV strain and FC. Figure 3 demonstrates a linear increase of FC probability with lower values of ΔRV strain; based on the fitted spline curve, the threshold value of 4% was chosen to discriminate FC vs. HCM. This cut-off value (ΔRV strain <4%) was also confirmed by the ROC analysis [sensitivity 68%, specificity 65%, positive predictive value (PPV) 33%, negative predictive value (NPV) 89%] (see Supplementary data online, Figure S1). The ROC analysis built for RV-FWS identified a value of 23.3% as the best threshold for discriminating FC vs. HCM (sensitivity 75%, specificity 72%, PPV 40%, NPV 92%). Notably, this threshold corresponded approximately to the lower limit of normality for RV-FWS (23.0%), derived from the literature (see Supplementary data online, Figure S1 and Table S4).
Table 2.
Multivariable logistic regression to identify parameters of RV mechanics independently associated with FC
| Echocardiographic parameters | Adjusted odds ratioa (95% CI) | P-value | C-statistic (95% CI) |
|---|---|---|---|
| RV-GLS (%) | 0.911 (0.805–1.031) | 0.141 | 0.848 (0.776–0.920) |
| RV-FWS (%) | 0.854 (0.766–0.953) | 0.005 | 0.870 (0.801–0.934) |
| ΔRV strain (%) | 0.676 (0.528–0.866) | 0.002 | 0.881 (0.809–0.953) |
Adjusted for IVS/PW thickness ratio, mitral SAM and RV wall thickness. Statistically signifcance at the 0.05 level is shown in bold type.
IVS/PW, interventricular septum/posterior wall.
Figure 3.
Spline curve analysis demonstrating the probability of FC according to ΔRV strain values. The spline curve demonstrates the changes of FC probability in phenotype-positive patients across the values of ΔRV strain, with overlaid % confidence intervals displayed (shaded areas).
Phenotype-negative patients
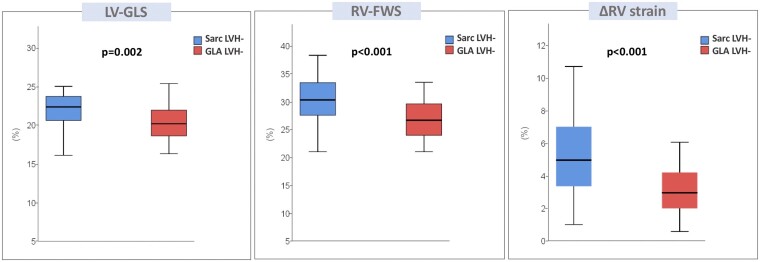
A total of 84 patients without LVH were included (28 GLA LVH- and 56 Sarc LVH-) (Table 3). As expected, most of the conventional echocardiographic parameters were within the normal range and did not significantly differ between the two groups, except for the E/e′ ratio that was higher in the Sarc LVH- group (P = 0.001). GLA LVH- patients showed significantly lower values of LV-GLS (P < 0.001). No significant differences were found in conventional parameters of RV systolic function between the two populations. However, when assessing RV systolic function by strain analysis, GLA LVH- patients showed significantly reduced RV-FWS (P < 0.0001) with lower values of ΔRV strain (P < 0.001), as compared to Sarc LVH- patients (Figure 4). Impaired RV-FWS values (defined as <23%) were found in 21% of GLA LVH- patients, as compared to 5% of Sarc LVH- patients (P = 0.025).
Table 3.
Clinical and echocardiographic characteristics of the phenotype-negative population
| Total LVH- (N = 84) | Sarc LVH- (N = 56) | GLA LVH- (N = 28) | P-value* | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| ȃAge (years) | 39 ± 15 | 37 ± 15 | 40 ± 15 | 0.337 |
| ȃFemale (n,%) | 57 (68) | 38 (68) | 19 (68) | 1.000 |
| ȃHypertension (n,%) | 15 (18) | 9 (16) | 6 (21) | 0.546 |
| Echocardiographic characteristics | ||||
| ȃLV mass index (g/m2) | 80 (68–93) | 80 (69–92) | 78 (66–93) | 0.708 |
| ȃLVEDV index (mL/m2) | 49 (43–54) | 51 (45–56) | 47 (40–52) | 0.256 |
| ȃLVEF (%) | 63 ± 5 | 63 ± 6 | 64 ± 3 | 0.258 |
| ȃLV-GLS (%) | 21.8 (19.9–23.2) | 22.3 (20.6–23.7) | 20.2 (18.5–22.0) | 0.002 |
| ȃLAV index (mL/m2) | 28 (23–32) | 27 (23–33) | 29 (24–30) | 1.000 |
| ȃE/A ratio | 1.3 (1.1–1.7) | 1.3 (1.1–1.5) | 1.6 (1.3–1.9) | 0.013 |
| ȃE/E′ ratio | 7.1 (5.8–8.6) | 7.9 (6.0–9.3) | 6.3 (5.4–7.3) | 0.001 |
| ȃRVWT (mm) | 3.5 (3.0–4.0) | 3.5 (3.0–4.0) | 3.6 (3.2–4.0) | 0.747 |
| ȃRVEDA (cm2) | 16.4 (14.2–19.3) | 16.9 (14.1–19.3) | 15.1 (14.5–18.6) | 0.407 |
| ȃRVESA (cm2) | 8.3 (6.5–9.7) | 8.3 (6.5–9.7) | 8.0 (6.3–10.6) | 0.859 |
| ȃRAV index (ml/m2) | 14 (11–19) | 15 (11–19) | 14 (11–19) | 0.772 |
| ȃTAPSE (mm) | 24 ± 3 | 24 ± 3 | 23 ± 3 | 0.745 |
| ȃRVFAC (%) | 51 ± 6 | 51 ± 6 | 51 ± 6 | 0.817 |
| ȃRV S′ velocity (cm/s) | 13.4 ± 2.2 | 13.2 ± 3.0 | 13.5 ± 1.5 | 0.669 |
| ȃRV-GLS (%) | 24.9 (22.2–27.4) | 25.3 (22.9–28.0) | 23.5 (21.8–26.0) | 0.049 |
| ȃRV-FWS (%) | 29.2 (26.6–32.6) | 30.3 (27.5–33.4) | 26.6 (23.9–29.6) | <0.001 |
| ȃΔRV strain (%) | 4.3 (2.8–5.9) | 5.0 (3.3–7.0) | 2.9 (2.0–4.3) | <0.001 |
| ȃTR jet velocity (m/s) | 2.1 (2.0–2.3) | 2.1 (2.0–2.3) | 2.1 (2.0–2.4) | 0.989 |
Comparison of echocardiographic parameters was performed applying a Bonferroni correction. P values <0.0028 were considered statistically significant (18 comparisons) and are shown in bold type.
LAV, left atrial volume; LVEDV, LV end diastolic volume; RAV, right atrial volume; RVEDA, RV end-diastolic area; RVESA, RV end-systolic area; RVWT, RV wall thickness; TR, tricuspid regurgitation.
Figure 4.
Box plots showing parameters of LV and RV strain in phenotype-negative patients. Lower, middle, and upper hinges of the box correspond to the 25th, 50th, and 75th percentiles. The upper and lower whiskers extend from the hinge to the largest and smallest value, respectively, no further than 1.5 times the interquartile range of the hinge.
Reproducibility
The intra- and inter-observer reproducibility of RV strain parameters is summarized in Supplementary data online, Table S5. The agreement was excellent for RV-GLS and RV-FWS and good for ΔRV strain measurements.
Discussion
In the present study we performed a comparative analysis of RV longitudinal mechanics assessed by 2D-STE in FC vs. HCM, as well as in genotype positive-phenotype negative patients. The original and relevant finding of the current study is that the impairment of RV strain in patients with FC shows peculiar features as compared to individuals with HCM and similar degree of LVH. Patients with FC exhibited a more prominent impairment of RV-FWS and lower values of ΔRV strain than HCM patients. This pattern was useful in discriminating FC from HCM and may be used as an adjunctive tool in the echocardiographic differential diagnosis between these two diseases. Moreover, we found that patients with Fabry disease without LVH have more reduced longitudinal strain values, as compared to patients with sarcomeric mutations without LVH, both at LV and RV level.
Phenotype-positive patients
RVH is considered a ‘red flag’ for HCM phenocopies.19 RVH has been reported with a prevalence ranging between 40 and 70% in patients with FC,2–4 and its presence has been shown to correlate with LVH, global disease severity and increasing age, suggesting that it is a feature of advanced stages of FC.2–4 In HCM, RVH is usually less frequent and milder as compared to storage/infiltrative cardiomyopathies5–7 but is still correlated with clinical and echocardiographic features of severe disease, especially LVH.5–7 In both diseases, RVH is typically associated with normal RV systolic function when assessed by standard echocardiography.3,4,6–8 However, the complex RV shape and geometry weaken the accuracy of conventional parameters of RV systolic function and recent studies on RV strain analysis revealed that this novel technique can unveil a common subclinical impairment of RV mechanics in both HCM and FC patients.7,9–12 In the present study, RV strain impairment was confirmed in a sizeable proportion of the cohort and was more frequent in FC than in HCM patients with similar degree of LVH. However, data on segmental variability of RV strain in cardiomyopathies with hypertrophic phenotype are still limited.17 In patients with FC, we previously reported that both RV-GLS and RV-FWS are more impaired in comparison to patients with Fabry disease in the pre-hypertrophic stage and healthy controls, while the equilibrium of RV mechanical properties was preserved, as demonstrated by similar values of ΔRV strain.10 Intriguingly, in the present study, FC patients showed a more prominent reduction of RV-FWS and lower values of ΔRV strain than HCM patients, adjusting for the potential confounding effect of the degree of LVH.
A previous work by Militaru et al.20showed a larger impairment of RV-FWS in 20 FC patients vs. 20 HCM patients with similar age and degree of LVH. The present study extended these findings in a larger and more homogenous population (only patients with genetically-proven, non-obstructive HCM were selected) and using a different post-processing software for strain measurements.
We can speculate that in FC the accumulation of globotriaosylceramide in the RV free-wall parallels LV structural changes, while in HCM the higher values of ΔRV strain are likely the consequence of the imbalance between the interventricular septum strain, which is more impaired, and RV free-wall strain, which is less affected. Notably, there was no difference in the tricuspid regurgitant jet velocity between the two populations, thus suggesting that afterload conditions do not play a major role in determining these differences.
Furthermore, RV-FWS and ΔRV strain showed an independent and incremental value in discriminating FC from HCM, above the presence of symmetric LVH, mitral SAM and RVH. Several echocardiographic features have been identified as evocative of FC rather than HCM, including the concentric pattern of LVH, disproportionate hypertrophy of papillary muscles, loss of base-to-apex circumferential strain gradient,1,19,21,22 but with variable sensitivity and specificity and none of them can be considered pathognomonic.1 In this scenario, our findings may have relevant implications in clinical practice, helping in the differential diagnosis. Nevertheless, our results do not allow to draw definite conclusions on the diagnostic value of RV strain impairment in the setting of unexplained LVH, since other HCM phenocopies (i.e. cardiac amyloidosis and Danon disease) have not been investigated in the present study. Additionally, although echocardiography remains the first-line investigation in routine clinical practice, it is worth mentioning the central role gained by cardiac magnetic resonance imaging with T1 mapping in the early differential diagnosis of FC.1
Phenotype-negative patients
Currently, no data are available on comparative echocardiographic analysis between sarcomeric vs. GLA pathogenic gene mutation carriers without LVH. Previous studies on pre-hypertrophic stages of both HCM and FD were mainly focused on the LV. Specifically, in carriers of HCM-related gene mutations, structural LV abnormalities have been described, including myocardial crypts and anterior mitral valve leaflet elongation,13 with conflicting results on LV function assessed by TDI and myocardial strain analysis.23,24 Conversely, patients with Fabry disease showed reduced TDI systolic velocities and LV-GLS values as compared to healthy controls.25–27 In the current study, patients with Fabry disease in the pre-hypertrophic stage showed worse LV-GLS values as compared to patients with sarcomeric mutations, even if LV-GLS was within the normal range in both populations. As regards the RV, the results obtained in the phenotype-positive populations were also confirmed in phenotype-negative patients. Indeed, RV-FWS and ΔRV strain values were lower in Fabry patients. This finding suggests that RV mechanical properties in Fabry patients are not entirely dependent upon RVH. We can hypothesize that, in the pre-hypertrophic stage of Fabry disease, a mild effect of globotriaosylceramide storage on both LV and RV may be detected by strain analysis, while in patients with HCM-related gene mutations, LV and RV mechanics remain unaffected.
Limitations
Some limitations of the present study should be acknowledged. First, given its retrospective nature, the study is not immune to source of bias. The HCM group was selected by matching with FC cases and therefore may be not representative of an average HCM population. Moreover, the majority of HCM patients had a mutation in the MYBPC3 gene and, although there were no significant differences between these patients and those with other sarcomeric gene mutations, further studies should be performed to clarify this issue.
Considering the unpredictable and variable penetrance of sarcomeric HCM, carriers of sarcomeric mutations that will never express the clinical phenotype may have been included in the present study. Similarly, in patients with a pre-hypertrophic stage of Fabry disease, the evolution of cardiac phenotype is not predictable, especially in females and with possible differences between naïve vs. treated patients.
In addition, cardiac magnetic resonance imaging was not systematically available and therefore the correlation between strain measurements and myocardial tissue characterization was not possible. Similarly, 3D echocardiographic datasets were not systematically acquired, thus 3D volumetric and strain measurements could not be performed.
Conclusions
Patients with FC have a more prominent reduction of RV-FWS and lower values of ΔRV strain as compared to HCM patients with similar degree of LVH. Both RV-FWS and ΔRV strain show an independent and incremental value in discriminating FC from sarcomeric HCM above conventional echocardiographic ‘red flags’.
Supplementary Material
Acknowledgements
The authors thank the Minister of Health RIcerca Corrente 2021 for the support.
Contributor Information
Maria Chiara Meucci, Department of Cardiology, Leiden University Medical Center, Albinusdreef 2 2300 RC, Leiden, The Netherlands; Catholic University of the Sacred Heart, Largo F. Vito 1, Rome 00168, Italy.
Rosa Lillo, Catholic University of the Sacred Heart, Largo F. Vito 1, Rome 00168, Italy; Department of Emergency Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Largo A. Gemelli 8, 00168 Rome, Italy.
Antonella Lombardo, Catholic University of the Sacred Heart, Largo F. Vito 1, Rome 00168, Italy; Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Largo A. Gemelli 8, 00168 Rome, Italy.
Gaetano A Lanza, Catholic University of the Sacred Heart, Largo F. Vito 1, Rome 00168, Italy; Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Largo A. Gemelli 8, 00168 Rome, Italy.
Marianne Bootsma, Department of Cardiology, Leiden University Medical Center, Albinusdreef 2 2300 RC, Leiden, The Netherlands.
Steele C Butcher, Department of Cardiology, Leiden University Medical Center, Albinusdreef 2 2300 RC, Leiden, The Netherlands; Department of Cardiology, Royal Perth Hospital, Victoria Square, 6000 Perth WA, Australia.
Massimo Massetti, Catholic University of the Sacred Heart, Largo F. Vito 1, Rome 00168, Italy; Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Largo A. Gemelli 8, 00168 Rome, Italy.
Raffaele Manna, Catholic University of the Sacred Heart, Largo F. Vito 1, Rome 00168, Italy; Institute of Internal Medicine, Periodic Fever and Rare Diseases Research Centre, Fondazione Policlinico A. Gemelli IRCCS, Largo A. Gemelli 8, 00168 Rome, Italy.
Jeroen J Bax, Department of Cardiology, Leiden University Medical Center, Albinusdreef 2 2300 RC, Leiden, The Netherlands; Heart Center, University of Turku and Turku University Hospital, Kiinamyllynkatu 4-8, 20521 Turku, Finland.
Filippo Crea, Catholic University of the Sacred Heart, Largo F. Vito 1, Rome 00168, Italy; Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Largo A. Gemelli 8, 00168 Rome, Italy.
Nina Ajmone Marsan, Department of Cardiology, Leiden University Medical Center, Albinusdreef 2 2300 RC, Leiden, The Netherlands.
Francesca Graziani, Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Largo A. Gemelli 8, 00168 Rome, Italy.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Funding
S.C.B. received funding from European Society of Cardiology (ESC Research Grant App000080404).
Data availability
The data that support the results of this study are available from the corresponding author upon reasonable request.
References
- 1. Pieroni M, Moon JC, Arbustini E, Barriales-Villa R, Camporeale A, Vujkovac ACet al. . Cardiac involvement in Fabry disease: JACC review topic of the week. J Am Coll Cardiol 2021;23:922–36. [DOI] [PubMed] [Google Scholar]
- 2. Niemann M, Breunig F, Beer M, Herrmann S, Strotmann J, Hu Ket al. . The right ventricle in Fabry disease: natural history and impact of enzyme replacement therapy. Heart 2010;96:1915–9. [DOI] [PubMed] [Google Scholar]
- 3. Palecek T, Dostalova G, Kuchynka P, Karetova D, Bultas J, Elleder Met al. . Right ventricular involvement in Fabry disease. J Am Soc Echocardiogr 2008;21:1265–8. [DOI] [PubMed] [Google Scholar]
- 4. Graziani F, Laurito M, Pieroni M, Pennestrì F, Lanza GA, Coluccia Vet al. . Right ventricular hypertrophy, systolic function, and disease severity in Anderson-Fabry disease: an echocardiographic study. J Am Soc Echocardiogr 2017;30:282–91. [DOI] [PubMed] [Google Scholar]
- 5. Maron MS, Hauser TH, Dubrow E, Horst TA, Kissinger KV, Udelson JEet al. . Right ventricular involvement in hypertrophic cardiomyopathy. Am J Cardiol 2007;100:1293–8. [DOI] [PubMed] [Google Scholar]
- 6. McKenna WJ, Kleinebenne A, Nihoyannopoulos P, Foale R. Echocardiographic measurement of right ventricular wall thickness in hypertrophic cardiomyopathy: relation to clinical and prognostic features. J Am Coll Cardiol 1988;11:351–8. [DOI] [PubMed] [Google Scholar]
- 7. Roşca M, Călin A, Beladan CC, Enache R, Mateescu AD, Gurzun MMet al. . Right ventricular remodeling, its correlates, and its clinical impact in hypertrophic cardiomyopathy. J Am Soc Echocardiogr 2015;28:1329–38. [DOI] [PubMed] [Google Scholar]
- 8. Graziani F, Lillo R, Panaioli E, Pieroni M, Camporeale A, Verrecchia Eet al. . Prognostic significance of right ventricular hypertrophy and systolic function in Anderson-Fabry disease. ESC Heart Fail 2020;7:1605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris DA, Blaschke D, Canaan-Kühl S, Krebs A, Knobloch G, Walter TCet al. . Global cardiac alterations detected by speckle-tracking echocardiography in Fabry disease: left ventricular, right ventricular, and left atrial dysfunction are common and linked to worse symptomatic status. Int J Cardiovasc Imaging 2015;31:301–13. [DOI] [PubMed] [Google Scholar]
- 10. Lillo R, Graziani F, Panaioli E, Mencarelli E, Pieroni M, Camporeale Aet al. . Right ventricular strain in Anderson-Fabry disease. Int J Cardiol 2021;330:84–90. [DOI] [PubMed] [Google Scholar]
- 11. Hiemstra YL, Debonnaire P, Bootsma M, Schalij MJ, Bax JJ, Delgado Vet al. . Prevalence and prognostic implications of right ventricular dysfunction in patients with hypertrophic cardiomyopathy. Am J Cardiol 2019;124:604–12. [DOI] [PubMed] [Google Scholar]
- 12. Wu XP, Li YD, Wang YD, Zhang M, Zhu WW, Cai QZet al. . Impaired right ventricular mechanics at rest and during exercise are associated with exercise capacity in patients with hypertrophic cardiomyopathy. J Am Heart Assoc 2019;8:e011269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott Pet al. . AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2020;76:3022–55. [DOI] [PubMed] [Google Scholar]
- 14. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande Let al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 15. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen Tet al. . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–60. [DOI] [PubMed] [Google Scholar]
- 16. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran Ket al. . Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–788. [DOI] [PubMed] [Google Scholar]
- 17. Muraru D, Haugaa K, Donal E, Stankovic I, Voigt JU, Petersen SE, et al. . Right ventricular longitudinal strain in the clinical routine: a state-of-the-art review. Eur Heart J Cardiovasc Imaging 2022:898–912. [DOI] [PubMed] [Google Scholar]
- 18. Muraru D, Onciul S, Peluso D, Soriani N, Cucchini U, Aruta Pet al. . Sex- and method-specific reference values for right ventricular strain by 2-dimensional speckle-tracking echocardiography. Circ Cardiovasc Imagaging 2016;9:e003866. [DOI] [PubMed] [Google Scholar]
- 19. Rapezzi C, Arbustini E, Caforio AL, Charron P, Gimeno-Blanes J, Heliö Tet al. . Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC working group on myocardial and pericardial diseases. Eur Heart J 2013;34:1448–58. [DOI] [PubMed] [Google Scholar]
- 20. Militaru S, Jurcuț R, Adam R, Roşca M, Ginghina C, Popescu BA. Echocardiographic features of Fabry cardiomyopathy-comparison with hypertrophy-matched sarcomeric hypertrophic cardiomyopathy. Echocardiography 2019;36:2041–9 [DOI] [PubMed] [Google Scholar]
- 21. Niemann M, Liu D, Hu K, Herrmann S, Breunig F, Strotmann Jet al. . Prominent papillary muscles in Fabry disease: a diagnostic marker? Ultrasound Med Biol 2011;37:37–43. [DOI] [PubMed] [Google Scholar]
- 22. Labombarda F, Saloux E, Milesi G, Bienvenu B. Loss of base-to-apex circumferential strain gradient: a specific pattern of Fabry cardiomyopathy? Echocardiography 2017;34:504–10. [DOI] [PubMed] [Google Scholar]
- 23. Nagueh SF, McFalls J, Meyer D, Hill R, Zoghbi WA, Tam JWet al. . Tissue Doppler imaging predicts the development of hypertrophic cardiomyopathy in subjects with subclinical disease. Circulation 2003;108:395–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williams LK, Misurka J, Ho CY, Chan WX, Agmon Y, Seidman Cet al. . Multilayer myocardial mechanics in genotype-positive left ventricular hypertrophy-negative patients with hypertrophic cardiomyopathy. Am J Cardiol 2018;122:1754–60. [DOI] [PubMed] [Google Scholar]
- 25. Pieroni M, Chimenti C, Ricci R, Sale P, Russo MA, Frustaci A. Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation 2003;107:1978–84. [DOI] [PubMed] [Google Scholar]
- 26. Saccheri MC, Cianciulli TF, Lax JA, Gagliardi JA, Cáceres GL, Quarin AEet al. . Two-dimensional speckle tracking echocardiography for early detection of myocardial damage in young patients with Fabry disease. Echocardiography 2013;30:1069–77. [DOI] [PubMed] [Google Scholar]
- 27. Lu DY, Huang WM, Wang WT, Hung SC, Sung SH, Chen CHet al. . Reduced global longitudinal strain as a marker for early detection of Fabry cardiomyopathy. Eur Heart J Cardiovasc Imaging 2022;23:487–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the results of this study are available from the corresponding author upon reasonable request.