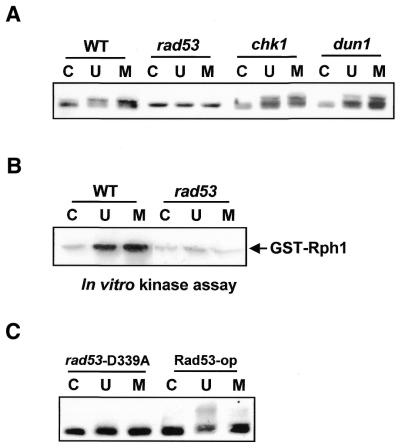
Figure 3.
Rph1 phosphorylation is dependent on Rad53 protein kinase. (A) Damage-dependent phosphorylation of Rph1 was checked in the rad53 defective mutant. Wild-type cell and rad53 mutant cell lysates were used for immunoblotting. (B) In vitro kinase assay was performed with GST–Rph1 fusion protein expressed in E.coli. GST–Rph1 proteins bound to glutathione–Sepharose were incubated with total cell lysates treated with UV (100 J/m2) or MMS (0.1%) at 30°C for 1 h and then the degree of phosphorylation was examined by using in vitro kinase assay. (C) Immunoblot analysis showed that Rph1 phosphorylation was diminished in rad53-D339A (a kinase dead mutant) but recovered in Rad53-op (Rad53-overexpressing). C, no damage; U, UV-irradiated (100 J/m2); M, MMS-treated (0.1%).