Abstract
Retinitis pigmentosa is a model for the study of genetic diseases. Its genetic heterogeneity is reflected in the different forms of inheritance (autosomal dominant, autosomal recessive, or X-linked) and, in a few families, in the presence of mutations in the visual pigment rhodopsin. Clinical and molecular genetic studies of these disorders are discussed. Animal models of retinal degeneration have been investigated for many years with the hope of gaining insight into the cause of photoreceptor cell death. Recently, the genes responsible for two of these animal disorders, the rds and rd mouse genes, have been isolated and characterized. The retinal degeneration of the rd mouse is presented in detail. The possible involvement of human analogues of these mouse genes in human retinal diseases is being investigated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Barrett D. J., Bateman J. B., Sparkes R. S., Mohandas T., Klisak I., Inana G. Chromosomal localization of human ornithine aminotransferase gene sequences to 10q26 and Xp11.2. Invest Ophthalmol Vis Sci. 1987 Jul;28(7):1037–1042. [PubMed] [Google Scholar]
- Berson E. L., Sandberg M. A., Rosner B., Birch D. G., Hanson A. H. Natural course of retinitis pigmentosa over a three-year interval. Am J Ophthalmol. 1985 Mar 15;99(3):240–251. doi: 10.1016/0002-9394(85)90351-4. [DOI] [PubMed] [Google Scholar]
- Bird A. C. X-linked retinitis pigmentosa. Br J Ophthalmol. 1975 Apr;59(4):177–199. doi: 10.1136/bjo.59.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker-Wagemakers L. M., Friedrich U., Gal A., Wienker T. F., Warburg M., Ropers H. H. Close linkage between Norrie disease, a cloned DNA sequence from the proximal short arm, and the centromere of the X chromosome. Hum Genet. 1985;71(3):211–214. doi: 10.1007/BF00284575. [DOI] [PubMed] [Google Scholar]
- Blodi F. C., Hunter W. S. Norrie's disease in North America. Doc Ophthalmol. 1969;26:434–450. doi: 10.1007/BF00944002. [DOI] [PubMed] [Google Scholar]
- Boughman J. A., Caldwell R. J. Genetic and clinical characterization of a survey population with retinitis pigmentosa. Prog Clin Biol Res. 1982;82:147–166. [PubMed] [Google Scholar]
- Boughman J. A., Conneally P. M., Nance W. E. Population genetic studies of retinitis pigmentosa. Am J Hum Genet. 1980 Mar;32(2):223–235. [PMC free article] [PubMed] [Google Scholar]
- Boughman J. A., Fishman G. A. A genetic analysis of retinitis pigmentosa. Br J Ophthalmol. 1983 Jul;67(7):449–454. doi: 10.1136/bjo.67.7.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes C., Danciger M., Kozak C. A., Farber D. B. Isolation of a candidate cDNA for the gene causing retinal degeneration in the rd mouse. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9722–9726. doi: 10.1073/pnas.86.24.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes C., Farber D. B. mRNAs coding for proteins of the cGMP cascade in the degenerative retina of the rd mouse. Exp Eye Res. 1987 Oct;45(4):467–480. doi: 10.1016/s0014-4835(87)80058-1. [DOI] [PubMed] [Google Scholar]
- Bowes C., Li T., Danciger M., Baxter L. C., Applebury M. L., Farber D. B. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990 Oct 18;347(6294):677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- Bowes C., van Veen T., Farber D. B. Opsin, G-protein and 48-kDa protein in normal and rd mouse retinas: developmental expression of mRNAs and proteins and light/dark cycling of mRNAs. Exp Eye Res. 1988 Sep;47(3):369–390. doi: 10.1016/0014-4835(88)90049-8. [DOI] [PubMed] [Google Scholar]
- Danciger M., Bowes C., Kozak C. A., LaVail M. M., Farber D. B. Fine mapping of a putative rd cDNA and its co-segregation with rd expression. Invest Ophthalmol Vis Sci. 1990 Aug;31(8):1427–1432. [PubMed] [Google Scholar]
- Danciger M., Farber D. B., Peyser M., Kozak C. A. The gene for the beta-subunit of retinal transducin (Gnb-1) maps to distal mouse chromosome 4, and related sequences map to mouse chromosomes 5 and 8. Genomics. 1990 Mar;6(3):428–435. doi: 10.1016/0888-7543(90)90472-7. [DOI] [PubMed] [Google Scholar]
- Danciger M., Kozak C. A., Farber D. B. The gene for the alpha-subunit of retinal rod transducin is on mouse chromosome 9. Genomics. 1989 Feb;4(2):215–217. doi: 10.1016/0888-7543(89)90303-0. [DOI] [PubMed] [Google Scholar]
- Danciger M., Kozak C. A., Li T., Applebury M. L., Farber D. B. Genetic mapping demonstrates that the alpha-subunit of retinal cGMP-phosphodiesterase is not the site of the rd mutation. Exp Eye Res. 1990 Aug;51(2):185–189. doi: 10.1016/0014-4835(90)90071-2. [DOI] [PubMed] [Google Scholar]
- Danciger M., Kozak C. A., Tsuda M., Shinohara T., Farber D. B. The gene for retinal S-antigen (48-kDa protein) maps to the centromeric portion of mouse chromosome 1 near Idh-1. Genomics. 1989 Aug;5(2):378–381. doi: 10.1016/0888-7543(89)90075-x. [DOI] [PubMed] [Google Scholar]
- Danciger M., Tuteja N., Kozak C. A., Farber D. B. The gene for the gamma-subunit of retinal cGMP-phosphodiesterase is on mouse chromosome 11. Exp Eye Res. 1989 Feb;48(2):303–308. doi: 10.1016/s0014-4835(89)80079-x. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., McGee T. L., Reichel E., Hahn L. B., Cowley G. S., Yandell D. W., Sandberg M. A., Berson E. L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990 Jan 25;343(6256):364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- FAVRE M. A propos de deux cas de dégénérescence hyaloidéorétinienne. Ophthalmologica. 1958 May-Jun;135(5-6):604–609. doi: 10.1159/000303360. [DOI] [PubMed] [Google Scholar]
- Farber D. B., Flannery J. G., Bird A. C., Shuster T., Bok D. Histopathological and biochemical studies on donor eyes affected with retinitis pigmentosa. Prog Clin Biol Res. 1987;247:53–67. [PubMed] [Google Scholar]
- Farber D. B., Lolley R. N. Cyclic guanosine monophosphate: elevation in degenerating photoreceptor cells of the C3H mouse retina. Science. 1974 Nov 1;186(4162):449–451. doi: 10.1126/science.186.4162.449. [DOI] [PubMed] [Google Scholar]
- Farber D. B., Lolley R. N. Enzymic basis for cyclic GMP accumulation in degenerative photoreceptor cells of mouse retina. J Cyclic Nucleotide Res. 1976;2(3):139–148. [PubMed] [Google Scholar]
- Farber D. B., Lolley R. N. Light-induced reduction in cyclic GMP of retinal photoreceptor cells in vivo: abnormalities in the degenerative diseases of RCS rats and rd mice. J Neurochem. 1977 May;28(5):1089–1095. doi: 10.1111/j.1471-4159.1977.tb10673.x. [DOI] [PubMed] [Google Scholar]
- Farber D. B., Park S., Yamashita C. Cyclic GMP-phosphodiesterase of rd retina: biosynthesis and content. Exp Eye Res. 1988 Mar;46(3):363–374. doi: 10.1016/s0014-4835(88)80026-5. [DOI] [PubMed] [Google Scholar]
- Farber D. B., Souza D. W., Chase D. G., Lolley R. N. Cyclic nucleotides of cone-dominant retinas. Reduction of cyclic AMP levels by light and by cone degeneration. Invest Ophthalmol Vis Sci. 1981 Jan;20(1):24–31. [PubMed] [Google Scholar]
- Fishman G. A., Kumar A., Joseph M. E., Torok N., Anderson R. J. Usher's syndrome. Ophthalmic and neuro-otologic findings suggesting genetic heterogeneity. Arch Ophthalmol. 1983 Sep;101(9):1367–1374. doi: 10.1001/archopht.1983.01040020369005. [DOI] [PubMed] [Google Scholar]
- Flannery J. G., Farber D. B., Bird A. C., Bok D. Degenerative changes in a retina affected with autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1989 Feb;30(2):191–211. [PubMed] [Google Scholar]
- Foxman S. G., Heckenlively J. R., Bateman J. B., Wirtschafter J. D. Classification of congenital and early onset retinitis pigmentosa. Arch Ophthalmol. 1985 Oct;103(10):1502–1506. doi: 10.1001/archopht.1985.01050100078023. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. K., Young J. H., Yamane H. K., Griswold-Prenner I. Subunit stoichiometry of retinal rod cGMP phosphodiesterase. Biochemistry. 1990 Mar 20;29(11):2657–2664. doi: 10.1021/bi00463a006. [DOI] [PubMed] [Google Scholar]
- Gal A., Stolzenberger C., Wienker T., Wieacker P., Ropers H. H., Friedrich U., Bleeker-Wagemakers L., Pearson P., Warburg M. Norrie's disease: close linkage with genetic markers from the proximal short arm of the X chromosome. Clin Genet. 1985 Mar;27(3):282–283. doi: 10.1111/j.1399-0004.1985.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Davies K. E., Ropers H. H. Report of the Committee on the Genetic Constitution of the X and Y Chromosomes. Cytogenet Cell Genet. 1985;40(1-4):296–352. doi: 10.1159/000132178. [DOI] [PubMed] [Google Scholar]
- Hansen A. C. Norrie's disease. Am J Ophthalmol. 1968 Aug;66(2):328–332. doi: 10.1016/0002-9394(68)92083-7. [DOI] [PubMed] [Google Scholar]
- Hayasaka S., Shiono T., Takaku Y., Mizuno K. Ornithine ketoacid aminotransferase in the bovine eye. Invest Ophthalmol Vis Sci. 1980 Dec;19(12):1457–1460. [PubMed] [Google Scholar]
- Heckenlively J. R., Foxman S. G., Parelhoff E. S. Retinal dystrophy and macular coloboma. Doc Ophthalmol. 1988 Mar-Apr;68(3-4):257–271. doi: 10.1007/BF00156432. [DOI] [PubMed] [Google Scholar]
- Heckenlively J. R. Preserved para-arteriole retinal pigment epithelium (PPRPE) in retinitis pigmentosa. Br J Ophthalmol. 1982 Jan;66(1):26–30. doi: 10.1136/bjo.66.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckenlively J. R. RP cone-rod degeneration. Trans Am Ophthalmol Soc. 1987;85:438–470. [PMC free article] [PubMed] [Google Scholar]
- Heckenlively J. R., Yoser S. L., Friedman L. H., Oversier J. J. Clinical findings and common symptoms in retinitis pigmentosa. Am J Ophthalmol. 1988 May 15;105(5):504–511. doi: 10.1016/0002-9394(88)90242-5. [DOI] [PubMed] [Google Scholar]
- Hill D. F., Chapman C. J., Gardner R. J. The use of a DNA marker for carrier diagnosis in an X-linked disorder: Norrie's disease. N Z Med J. 1987 Mar 25;100(820):166–168. [PubMed] [Google Scholar]
- Inana G., Totsuka S., Redmond M., Dougherty T., Nagle J., Shiono T., Ohura T., Kominami E., Katunuma N. Molecular cloning of human ornithine aminotransferase mRNA. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1203–1207. doi: 10.1073/pnas.83.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay M. On the heredity of retinitis pigmentosa. Br J Ophthalmol. 1982 Jul;66(7):405–416. doi: 10.1136/bjo.66.7.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberling W. J., Weston M. D., Möller C., Davenport S. L., Shugart Y. Y., Priluck I. A., Martini A., Milani M., Smith R. J. Localization of Usher syndrome type II to chromosome 1q. Genomics. 1990 Jun;7(2):245–249. doi: 10.1016/0888-7543(90)90546-7. [DOI] [PubMed] [Google Scholar]
- Kivlin J. D., Sanborn G. E., Wright E., Cannon L., Carey J. Further linkage data on Norrie disease. Am J Med Genet. 1987 Mar;26(3):733–736. doi: 10.1002/ajmg.1320260329. [DOI] [PubMed] [Google Scholar]
- LaVail M. M., Sidman R. L. C57BL-6J mice with inherited retinal degeneration. Arch Ophthalmol. 1974 May;91(5):394–400. doi: 10.1001/archopht.1974.03900060406015. [DOI] [PubMed] [Google Scholar]
- McWilliam P., Farrar G. J., Kenna P., Bradley D. G., Humphries M. M., Sharp E. M., McConnell D. J., Lawler M., Sheils D., Ryan C. Autosomal dominant retinitis pigmentosa (ADRP): localization of an ADRP gene to the long arm of chromosome 3. Genomics. 1989 Oct;5(3):619–622. doi: 10.1016/0888-7543(89)90031-1. [DOI] [PubMed] [Google Scholar]
- Meyer K. T., Heckenlively J. R., Spitznas M., Foos R. Y. Dominant retinitis pigmentosa. A clinicopathologic correlation. Ophthalmology. 1982 Dec;89(12):1414–1424. doi: 10.1016/s0161-6420(82)34631-x. [DOI] [PubMed] [Google Scholar]
- Musarella M. A., Burghes A., Anson-Cartwright L., Mahtani M. M., Argonza R., Tsui L. C., Worton R. Localization of the gene for X-linked recessive type of retinitis pigmentosa (XLRP) to Xp21 by linkage analysis. Am J Hum Genet. 1988 Oct;43(4):484–494. [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Piantanida T. P., Eddy R. L., Shows T. B., Hogness D. S. Molecular genetics of inherited variation in human color vision. Science. 1986 Apr 11;232(4747):203–210. doi: 10.1126/science.3485310. [DOI] [PubMed] [Google Scholar]
- Ngo J. T., Bateman J. B., Cortessis V., Sparkes R. S., Mohandas T., Inana G., Spence M. A. Norrie disease: linkage analysis using a 4.2-kb RFLP detected by a human ornithine aminotransferase cDNA probe. Genomics. 1989 May;4(4):539–545. doi: 10.1016/0888-7543(89)90277-2. [DOI] [PubMed] [Google Scholar]
- Ngo J. T., Bateman J. B., Spence M. A., Cortessis V., Sparkes R. S., Kivlin J. D., Mohandas T., Inana G. Ornithine aminotransferase (OAT): recombination between an X-linked OAT sequence (7.5 kb) and the Norrie disease locus. Genomics. 1990 Jan;6(1):123–128. doi: 10.1016/0888-7543(90)90456-5. [DOI] [PubMed] [Google Scholar]
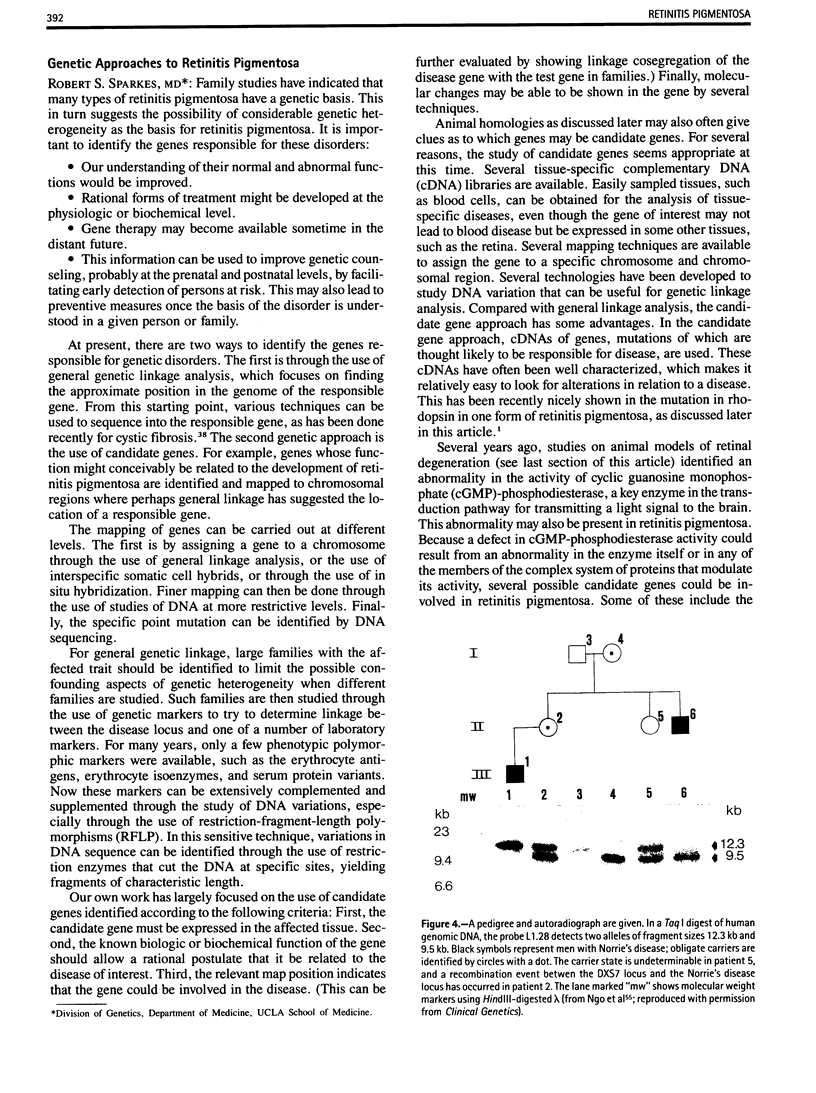
- Ngo J. T., Spence M. A., Cortessis V., Sparkes R. S., Bateman J. B. Recombinational event between Norrie disease and DXS7 loci. Clin Genet. 1988 Jul;34(1):43–47. doi: 10.1111/j.1399-0004.1988.tb02614.x. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Lowry O. H., Cohen A. I., Ferrendelli J. A. Distribution of 3':5'-cyclic AMP and 3':5'-cyclic GMP in rabbit retina in vivo: selective effects of dark and light adaptation and ischemia. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4442–4445. doi: 10.1073/pnas.73.12.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K., Farber D. B., Hargrave P. A. Elevated level of protein phosphatase 2A activity in retinas of rd mice. Exp Eye Res. 1991 Jul;53(1):101–105. doi: 10.1016/0014-4835(91)90151-4. [DOI] [PubMed] [Google Scholar]
- Ramesh V., Eddy R., Bruns G. A., Shih V. E., Shows T. B., Gusella J. F. Localization of the ornithine aminotransferase gene and related sequences on two human chromosomes. Hum Genet. 1987 Jun;76(2):121–126. doi: 10.1007/BF00284906. [DOI] [PubMed] [Google Scholar]
- Rommens J. M., Iannuzzi M. C., Kerem B., Drumm M. L., Melmer G., Dean M., Rozmahel R., Cole J. L., Kennedy D., Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989 Sep 8;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Ross D. F., Fishman G. A., Gilbert L. D., Anderson R. J. Variability of visual field measurements in normal subjects and patients with retinitis pigmentosa. Arch Ophthalmol. 1984 Jul;102(7):1004–1010. doi: 10.1001/archopht.1984.01040030806021. [DOI] [PubMed] [Google Scholar]
- SIDMAN R. L., GREEN M. C. RETINAL DEGENERATION IN THE MOUSE: LOCATION OF THE RD LOCUS IN LINKAGE GROUP XVII. J Hered. 1965 Jan-Feb;56:23–29. doi: 10.1093/oxfordjournals.jhered.a107364. [DOI] [PubMed] [Google Scholar]
- Schachat A. P., Maumenee I. H. Bardet-Biedl syndrome and related disorders. Arch Ophthalmol. 1982 Feb;100(2):285–288. doi: 10.1001/archopht.1982.01030030287011. [DOI] [PubMed] [Google Scholar]
- Shuster T. A., Farber D. B. Phosphorylation in sealed rod outer segments: effects of cyclic nucleotides. Biochemistry. 1984 Jan 31;23(3):515–521. doi: 10.1021/bi00298a018. [DOI] [PubMed] [Google Scholar]
- Shuster T. A., Farber D. B. Rhodopsin phosphorylation in developing normal and degenerative mouse retinas. Invest Ophthalmol Vis Sci. 1986 Feb;27(2):264–268. [PubMed] [Google Scholar]
- Sparkes R. S., Klisak I., Kaufman D., Mohandas T., Tobin A. J., McGinnis J. F. Assignment of the rhodopsin gene to human chromosome three, region 3q21-3q24 by in situ hybridization studies. Curr Eye Res. 1986 Oct;5(10):797–798. doi: 10.3109/02713688609000021. [DOI] [PubMed] [Google Scholar]
- Sunga R. N., Sloan L. L. Pigmentary degeneration of the retina: early diagnosis and natural history. Invest Ophthalmol. 1967 Jun;6(3):309–325. [PubMed] [Google Scholar]
- Takki K. Gyrate atrophy of the choroid and retina associated with hyperornithinaemia. Br J Ophthalmol. 1974 Jan;58(1):3–23. doi: 10.1136/bjo.58.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis G. H., Brennan M. B., Danielson P. E., Kozak C. A., Sutcliffe J. G. Identification of a photoreceptor-specific mRNA encoded by the gene responsible for retinal degeneration slow (rds). Nature. 1989 Mar 2;338(6210):70–73. doi: 10.1038/338070a0. [DOI] [PubMed] [Google Scholar]
- Tuteja N., Tuteja R., Farber D. B. Cloning and sequencing of the gamma-subunit of retinal cyclic-GMP phosphodiesterase from rd mouse. Exp Eye Res. 1989 Jun;48(6):863–872. doi: 10.1016/0014-4835(89)90069-9. [DOI] [PubMed] [Google Scholar]
- Valle D., Kaiser-Kupfer M. I., Del Valle L. A. Gyrate atrophy of the choroid and retina: deficiency of ornithine aminotransferase in transformed lymphocytes. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5159–5161. doi: 10.1073/pnas.74.11.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon M. Usher's syndrome--deafness and progressive blindness. Clinical cases, prevention, theory and literature survey. J Chronic Dis. 1969 Aug;22(3):133–151. doi: 10.1016/0021-9681(69)90055-1. [DOI] [PubMed] [Google Scholar]
- WARBURG M. Norie's disease (atrofia bulborum hereditaria). Acta Ophthalmol (Copenh) 1963;41:134–146. doi: 10.1111/j.1755-3768.1963.tb03533.x. [DOI] [PubMed] [Google Scholar]
- Wieacker P., Davies K. E., Cooke H. J., Pearson P. L., Williamson R., Bhattacharya S., Zimmer J., Ropers H. H. Toward a complete linkage map of the human X chromosome: regional assignment of 16 cloned single-copy DNA sequences employing a panel of somatic cell hybrids. Am J Hum Genet. 1984 Mar;36(2):265–276. [PMC free article] [PubMed] [Google Scholar]
- Young R. W. Solar radiation and age-related macular degeneration. Surv Ophthalmol. 1988 Jan-Feb;32(4):252–269. doi: 10.1016/0039-6257(88)90174-9. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A., Sankila E. M., Lindlöf M., Aula P., Norio R. Norrie disease caused by a gene deletion allowing carrier detection and prenatal diagnosis. Clin Genet. 1985 Oct;28(4):317–320. doi: 10.1111/j.1399-0004.1985.tb00405.x. [DOI] [PubMed] [Google Scholar]