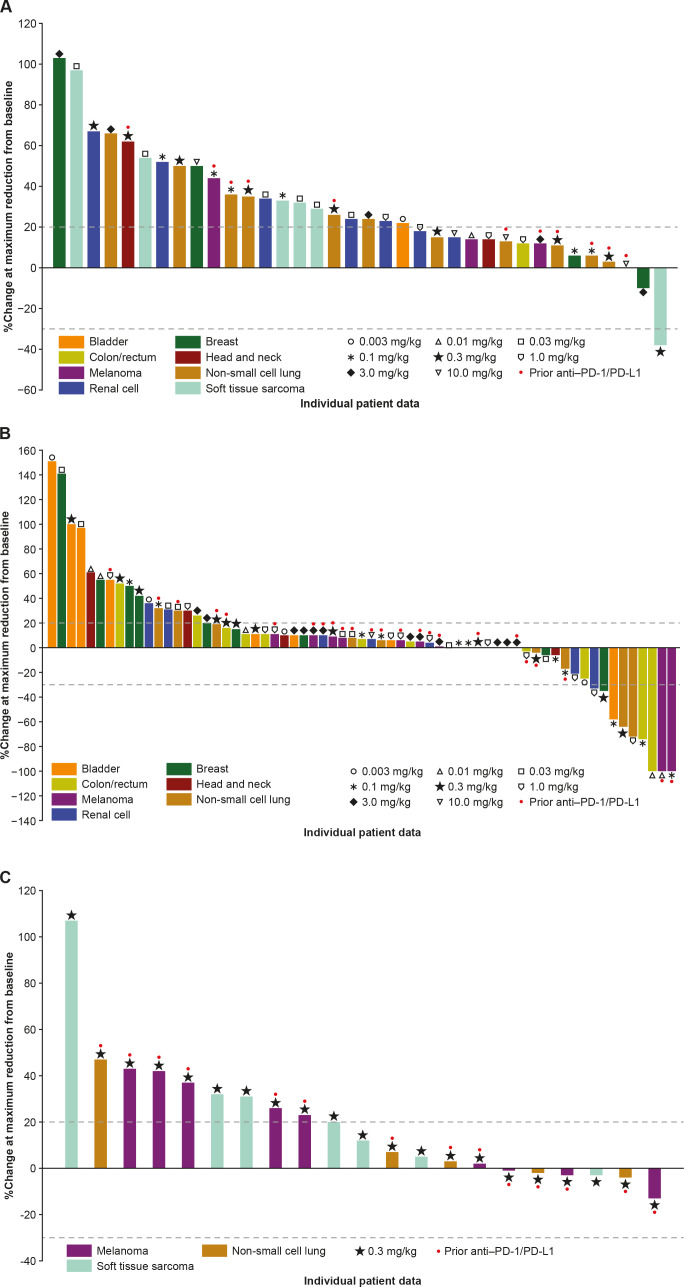
Figure 2.
Percentage change in sum of target lesion diameters from baseline in (A) Part 1 dose escalation (GSK3174998 alone), (B) Part 2 dose escalation (GSK3174998+pembrolizumab), and (C) Part 2 dose expansion (GSK3174998+pembrolizumab; one patient with soft tissue sarcoma was not evaluable) assessed using irRECIST. irRECIST, immune-related Response Evaluation Criteria in Solid Tumors; PD-1, programmed cell death 1 protein; PD-L1, programmed cell death 1 ligand.